(UroToday.com) The 2024 GU ASCO annual meeting included a urothelial carcinoma session featuring trials in progress and a presentation by Dr. Shilpa Gupta discussing the trial design of MAIN-CAV, a phase III randomized trial of maintenance cabozantinib and avelumab vs avelumab after first-line platinum-based chemotherapy in patients with metastatic urothelial cancer. First-line induction platinum-based chemotherapy followed by switch maintenance avelumab is the current preferred standard of care in patients with advanced urothelial carcinoma who do not progress after platinum-based chemotherapy.1
However, there is a significant need to further improve outcomes by combining avelumab with an effective, non-cross resistant therapy with non-overlapping toxicity. Cabozantinib is an oral inhibitor of MET, VEGFR, and TAM family receptors involved in tumor growth, angiogenesis, and immune cell regulation and has shown efficacy in urothelial carcinoma in combination with PD-1/PD-1L1 inhibitor. Dr. Shilpa and colleagues hypothesized that cabozantinib + avelumab combination would improve survival vs avelumab alone and have an acceptable safety profile as switch maintenance therapy in metastatic urothelial carcinoma.
MAIN-CAV is a phase III randomized, multicenter, international trial for patients with locally advanced/metastatic urothelial carcinoma (including cN3M0 only)) who do not progress after 4-6 cycles of any platinum-based chemotherapy (gemcitabine-cisplatin, gemcitabine-carboplatin, MVAC, or ddMVAC). There will be 654 adults randomized 1:1 within 3-10 weeks after the last dose of chemotherapy to receive avelumab 800 mg IV every 2 weeks or avelumab and cabozantinib 40 mg daily for up to 2 years. Key eligibility criteria include:
- ECOG performance status 0-1
- No prior use of anti-PD(L)1
- No CNS metastases
- No major surgery within four weeks
- No uncontrolled hypertension or cardiovascular disorders
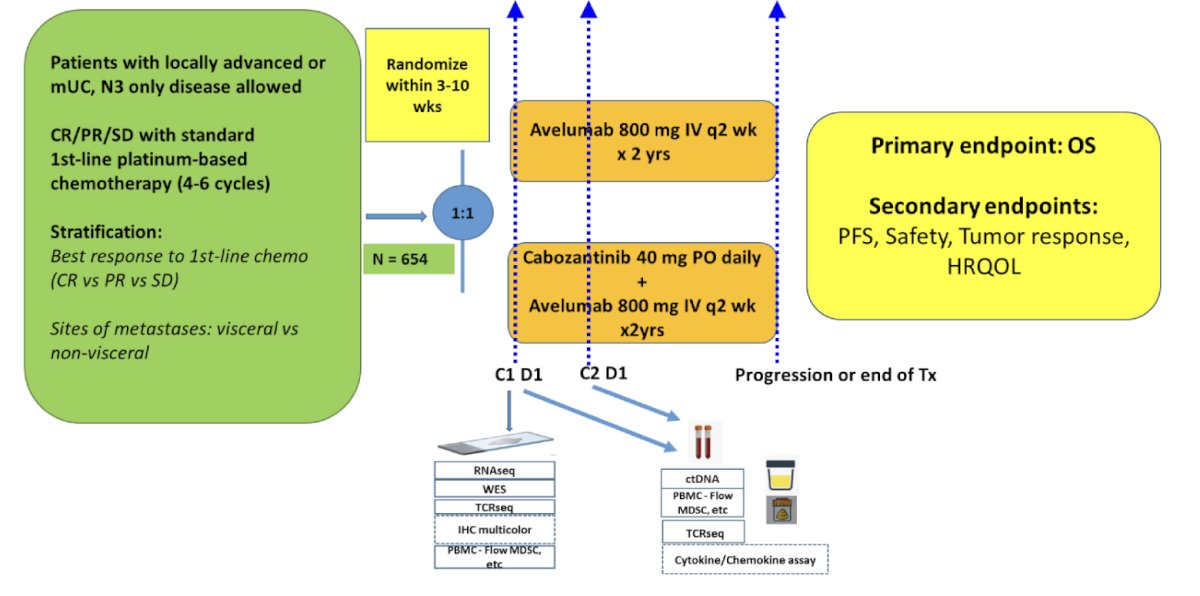
Patients are stratified based on 1) best response to first line chemotherapy: complete vs partial response vs stable disease and 2) presence vs absence of visceral metastases. The primary endpoint is overall survival with assumptions of 1-sided alpha 0.025, power 80%, median overall survival 21 months with avelumab, and hazard ratio 0.75, thus hypothesizing median overall survival 28 months on cabozantinib + avelumab. Key secondary endpoints include progression-free survival, safety, tolerability, and efficacy of cabozantinib + avelumab vs avelumab alone based on RECIST 1.1 and iRECIST criteria (and PD-L1 status). Quality of life are assessed using EQ-5D-5L, PROMIS-Fatigue 4a, EORTC QLQ-C30, EORTC QLQ-BLM30 between patients on cabozantinib + avelumab vs avelumab alone. Biomarkers of response and resistance to avelumab will be assessed using baseline archival tissues, baseline and serial blood, ctDNA, stool, and urine. Imaging studies will test the correlation of established and new radiomic signatures with survival, adverse events, and quality of life, and incorporate both radiologic and biologic features to assess their potential association with outcomes.
This trial would be the first to systematically address whether adding the multi-targeted TKI, cabozantinib, to avelumab improves survival vs avelumab alone as first line maintenance therapy.
Clinical trial information: NCT05092958.
Presented by: Shilpa Gupta, MD, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
Related content: Combination Maintenance Therapy for Advanced Bladder Cancer Explored in MAIN-CAV Study - Shilpa Gupta
References:
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.


