(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma poster session. Borivoj Golijanin presented the results of a study evaluating the adverse effects and discontinuation rates of pembrolizumab for BCG refractory non-muscle invasive bladder cancer (NMIBC).
Following the results of the KEYNOTE-057 trial that demonstrated a 3-months complete response rate of 41% that was maintained in approximately half of such patients at one year, pembrolizumab has become approved for patients with high-risk, BCG-refractory carcinoma in situ (+/- papillary tumors) who received adequate BCG therapy and are ineligible for or refusing radical cystectomy.1 This study reports on a single institutional experience (Brown University) using pembrolizumab.
This analysis included 18 patients with a median age of 74.5 years and a median follow-up of 17.5 months. Clinical stage at pembrolizumab start was cT1 in 61%, carcinoma in situ in 33%, and cTa in 6%.
On average, patients received 12.4 cycles. Of the 18 patients, 13 terminated pembrolizumab treatment early, mainly due to grade ≥2 toxicity (62%), but also disease progression (31%), and disease recurrence (8%).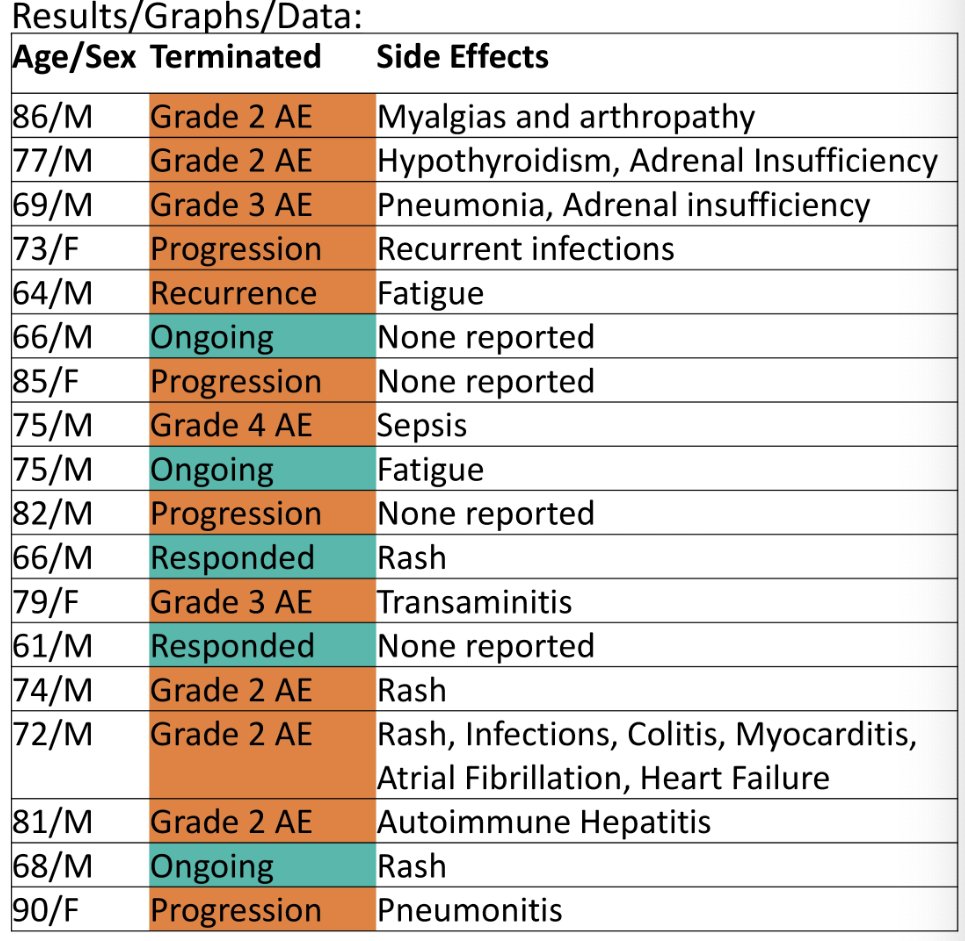
Based on these results, the investigators concluded that:
- Treatment with pembrolizumab was not well tolerated
- High toxicity leads to withdrawal from treatment
- These single institutional findings do not confirm those of KEYNOTE-057
- Additional research is needed to better identify patients who will benefit from this agent
Presented by: Borivoj Golijanin, BS, Research Fellow, The Minimally Invasive Urology Institute, Division of Urology, The Miriam Hospital, Warren Alpert Medical School of Brown University, Providence, RI
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:

