(UroToday.com) The 2024 GU ASCO annual meeting featured a urothelial carcinoma session and a presentation by Dr. Zongren Wang discussing preliminary results from the Punch phase II study of intra‑arterial chemotherapy combined with tislelizumab and BCG in high-risk non-muscle-invasive bladder cancer (NMIBC). Previous studies have shown that intra‑arterial chemotherapy could reduce the recurrence and progression of high risk NMIBC. Furthermore, the KEYNOTE-057 study has supported the benefits of PD-1 inhibitors in high risk NMIBC patients.1 This study was established to evaluate the efficacy and safety of intra‑arterial chemotherapy combined with tislelizumab and BCG as a bladder-preserving treatment for high risk NMIBC patients.
This open-label, single arm, phase II study enrolled BCG-naïve high risk NMIBC patients with papillary tumors (high-grade Ta or T1 tumors). First, all visible papillary lesions were removed by TURBT. Second, an angiographic catheter was placed into the internal iliac arteries with Seldinger’s percutaneous technique, cisplatin (60 mg/m2) and epirubicin (50 mg/m2) were administered arterially in day 1, and every 3 weeks for 2-4 cycles. Also, patients received tislelizumab 200 mg IVGTT on day 1, and every 3 weeks for 2-4 cycles. Finally, patients received 18 instillations of BCG plus at least 8 cycles of tislelizumab (200 mg IVGTT, every 3 weeks). Specifically, patients were started on an induction course of BCG with 6 instillations every week, followed by maintenance with 3 instillations every 2 weeks and 9 instillations every 4 weeks:
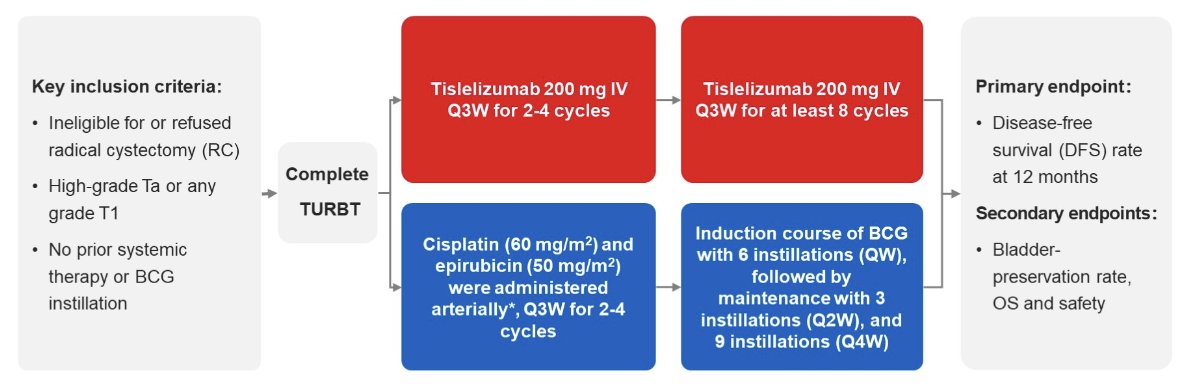
The primary endpoint was disease-free survival rate at 12 months (defined as no reappearance of high grade or T1 tumors or clinical stage development after the therapy). Secondary endpoints were bladder-preservation rate, overall survival, and safety. This study estimated a disease-free survival rate at 12 months was no less than 55% and the study would enroll 27 patients.
By December 2023, 15 eligible patients were enrolled, of which were 80% male, had a median age of 60 years (range: 39-79), 73.3% had pure urothelial carcinoma, median tumor size was 3.0 cm (range: 0.6-5.5), multiple papillary tumors were present in 60% of patients, and 40% were high-grade Ta and 60% were T1. The median follow-up was 13.9 months (range: 4.4-33.9), the mean number of intra‑arterial chemotherapy cycles was 2.6, the mean number of tislelizumab cycles was 9.5, and the median BCG instillations was 11 (range: 3-18). The baseline characteristics are as follows:

The disease-free survival rate at 12 months was 100% (95% CI, 78.2% - 100%), the bladder-preservation rate at 12 months was 100% (95% CI, 100% - 100%), and the overall survival rate at 12 months was 100% (95% CI, 100%-100%). Three patients experienced treatment related adverse events, including nausea (n = 1, grade 2), myalgia (n = 1, grade 2), fatigue (n = 1, grade 2), neutropenia (n = 1, grade 2) and fever (n = 1, grade 3).
Dr. Wang concluded this presentation by discussing preliminary results from the Punch phase II study of intra‑arterial chemotherapy combined with tislelizumab and BCG in high-risk NMIBC with the following take-home points:
- These preliminary results support the use of intra‑arterial chemotherapy combined with tislelizumab and BCG as a promising bladder-preserving strategy for high risk NMIBC patients
Presented by: Zongren Wang, Department of Urology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


