(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium included a urothelial carcinoma session featuring trials in progress and a presentation by Dr. Richard Cathomas discussing the trial design of SAKK 06/19, a phase 2 single arm trial assessing intravesical recombinant BCG followed by perioperative chemo-immunotherapy for patients with muscle-invasive bladder cancer. The integration of immune checkpoint inhibitors in the perioperative setting of localized muscle-invasive bladder cancer is extensively investigated and several results from phase 2 trials have been published. While promising, the pathological complete remission rate (ypT0 ypN0) achieved with immune checkpoint inhibitor-containing regimens appears similar to cisplatin-based chemotherapy alone. However, further improvement is needed. Intravesical BCG has successfully been used for decades for non-muscle invasive bladder cancer (NMIBC) in patients with carcinoma in situ (CIS) and as adjuvant treatment for high-risk papillary tumors. Intravesical BCG induces a local inflammation leading to induction of the innate immune system, probably followed by a tumor-specific adaptive immune response. Recently, a novel recombinant BCG vaccine (VPM1002BC) has been developed and a clinical study in BCG-refractory NMIBC1 has demonstrated good safety with low local toxicity and promising efficacy and promising cytokine pattern, including IFNy and GM-CSF:
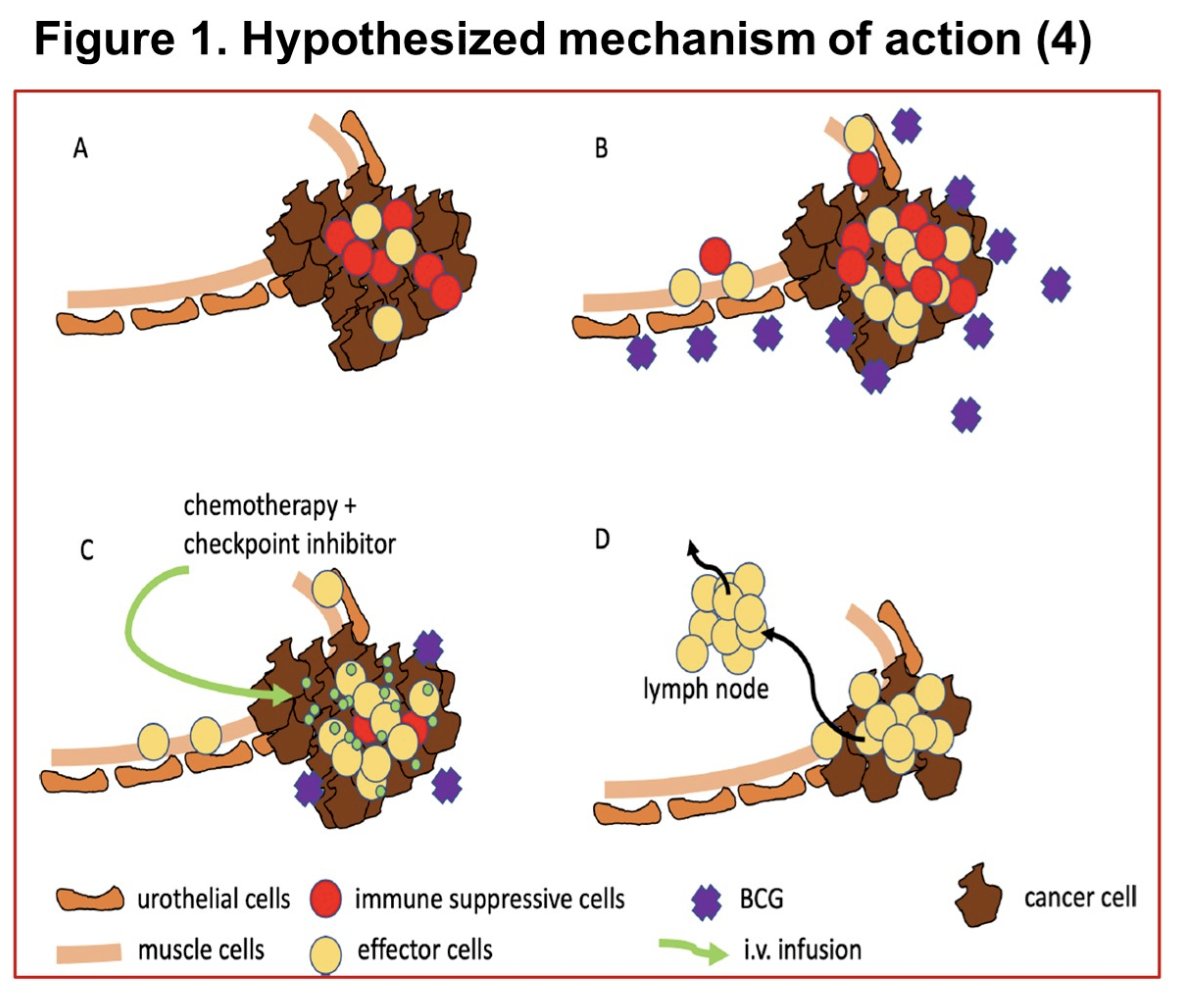
Dr. Cathomas and colleagues hypothesized that induction therapy with intravesical VPM1002BC improves the efficacy of systemic perioperative chemo-immunotherapy in patients with operable muscle-invasive bladder cancer.
SAKK 06/19 is an open-label, single arm, phase II trial for patients with operable pT2 or cT2-T4a cN0-1 muscle-invasive bladder cancer without contraindication for cisplatin. Prior intravesical BCG is excluded, as well as patients unable to keep BCG instillation for less than 1 hour. Intravesical VPM1002BC is administered weekly for 3 instillations. Atezolizumab 1200mg is also given on day 1 and then every 3 weeks for four times. Chemotherapy with cisplatin (70mg/m2 day 1) and gemcitabine (1000mg/m2 day 1 and 8) every 3 weeks is started on day 22 and given for four cycles followed by radical cystectomy and lymphadenectomy. Atezolizumab is continued after surgery for 13 cycles in case of residual muscle invasive disease (≥ypT2) or positive lymph nodes (ypN+) only. The trial design for SAKK 06/19 is as follows:
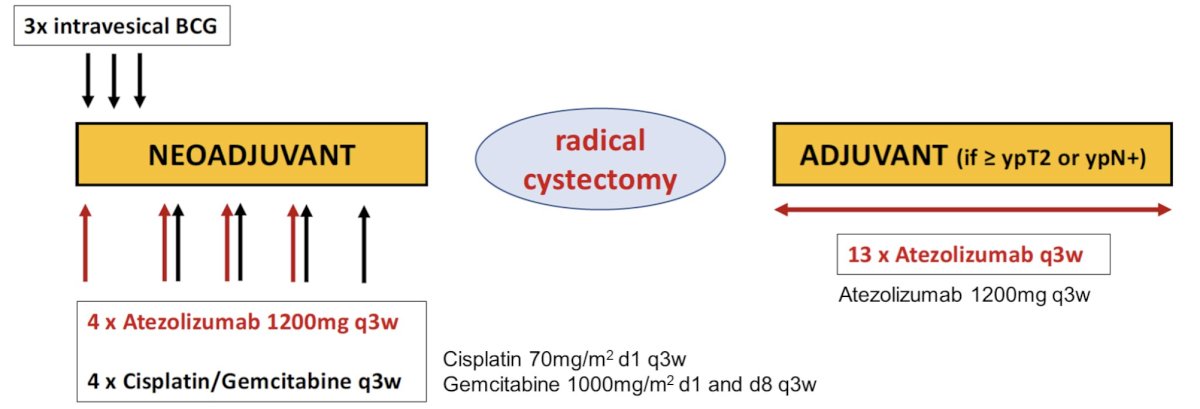
Pathologic complete response at cystectomy is the primary endpoint. A total of 46 patients are needed (including 15% dropout rate) using Simon’s minimax two-stage design with type I error 5%, power 80%, a null hypothesis of ≤35% pathologic complete response, and an alternative hypothesis of ≥55% pathologic complete response.
Secondary endpoints include:
- Pathological response rate (<ypT2N0)
- Event-free survival
- Recurrence-free survival
- Overall survival
- Feasibility
- Toxicity
An interim safety analysis will be performed after the first 12 patients have completed neoadjuvant treatment specifically assessing toxicity possibly associated with intravesical BCG application. An interim efficacy analysis will be performed after the first 21 patients have undergone surgery.
Additional research questions include:
- Preoperative assessment of treatment response using MRI, urine cytology, and circulating tumor DNA (ctDNA), and correlation with pathological outcome
- Postoperative ctDNA and correlation with time to event endpoints
- The tumor immunome before and after neoadjuvant therapy
- Biomarkers for anti-PD-L1 treatment and their relation to efficacy endpoints
- The effect of the gut microbiota on the response to immunotherapy
- Immune parameters in urine samples and their relation to efficacy endpoints
Accrual to the study is currently ongoing.
Clinical trial information: NCT04630730.
Presented by: Richard Cathomas, Division of Oncology, Cantonal Hospital Graubunden, Chur, Switzerland
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


