(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma poster session. Dr. Jiwei Huang presented the results of a phase II study of tislelizumab monotherapy as neoadjuvant treatment for cisplatin-ineligible patients with high-risk upper tract urothelial carcinoma (UTUC).
Platinum-based chemotherapy is standard of care following radical nephroureterectomy for high-risk UTUC patients, based on the results of the POUT trial.1 However, more than half of UTUC patients may suffer from severe renal function loss which limits the use of cisplatin. PD-1 checkpoint inhibitors, the dosing of which is not significantly limited by renal function and does not adversely impact renal function, has demonstrated preliminary efficacy in the neoadjuvant treatment of bladder cancer with 31% to 42% of patients achieving pathological complete response.2 In this study, Dr. Hwang presented the efficacy and safety results of tislelizumab monotherapy in the neoadjuvant treatment of patients with high-risk UTUC, who are ineligible for cisplatin-based chemotherapy.
Eligible patients had high-risk UTUC defined as:
- Pathological high-grade UTUC (either by endoscopic biopsy or urinary cytology) and/or
- Invasive aspect on radiological examination (cT2-TaN0-2MO) and/or hydronephrosis
All patients were required to have an ECOG performance status of 0-2, no prior systemic therapy, and be ineligible for cisplatin. Patients with variant histology on biopsy were included.
Patients were administered tislelizumab 200mg IV every 3 weeks for a maximum of 4 cycles followed by surgery (radical nephroureterectomy, ureteral resection, or endoscopic ablation). Contrast-enhanced MRI examination was performed prior to the third dose and again prior to surgery (Figure 1).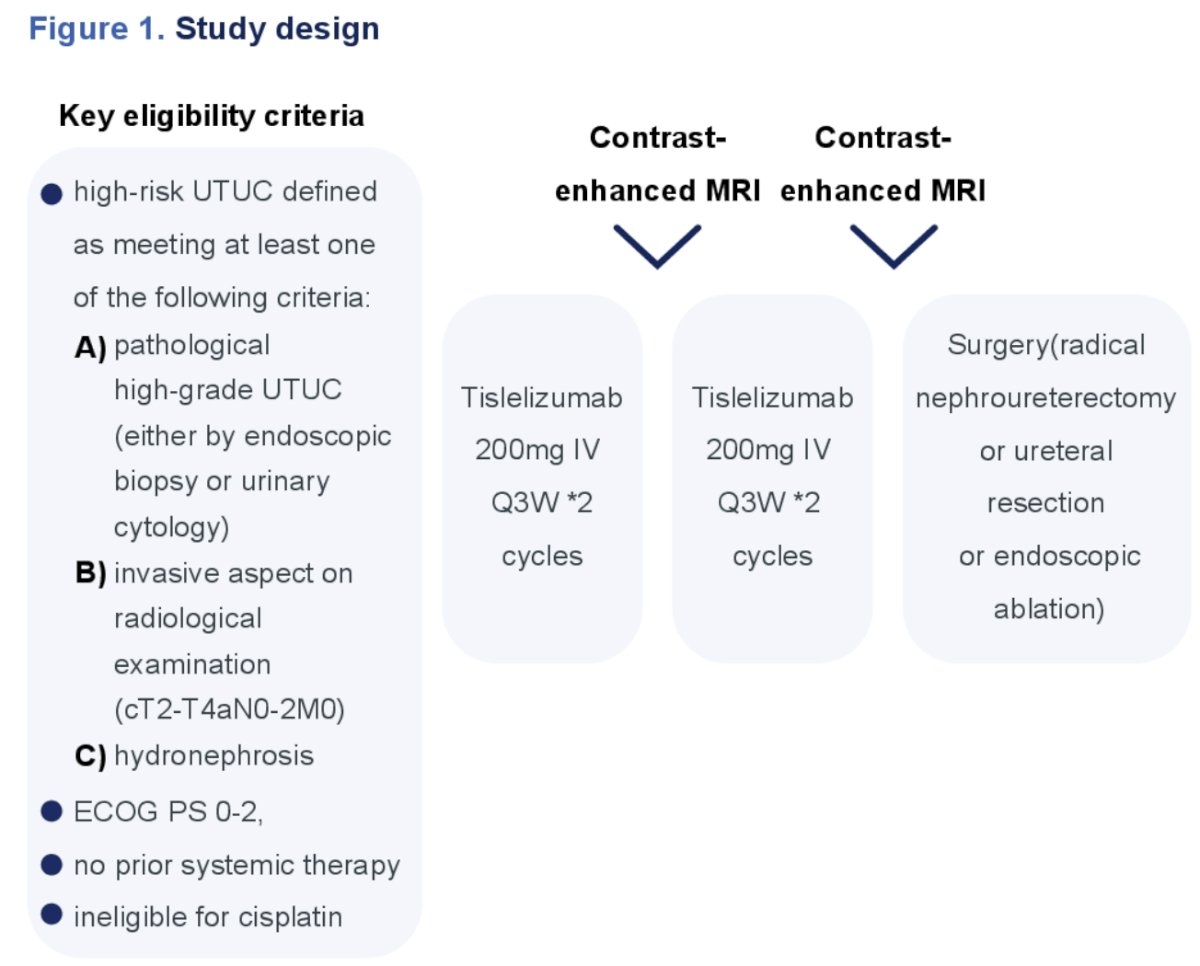
The primary endpoint was pathological complete response rate, defined as pT0N0. The secondary endpoints included:
- Pathological response rate (≤ypT1N0)
- Disease-free survival
- Safety
This study included 16 patients, recruited between March 2021 and September 2022. The baseline characteristics are summarized in Table 1. The median patient age was 65 years, 81% were male, 75% had cT3-4 disease, and 25% cN+.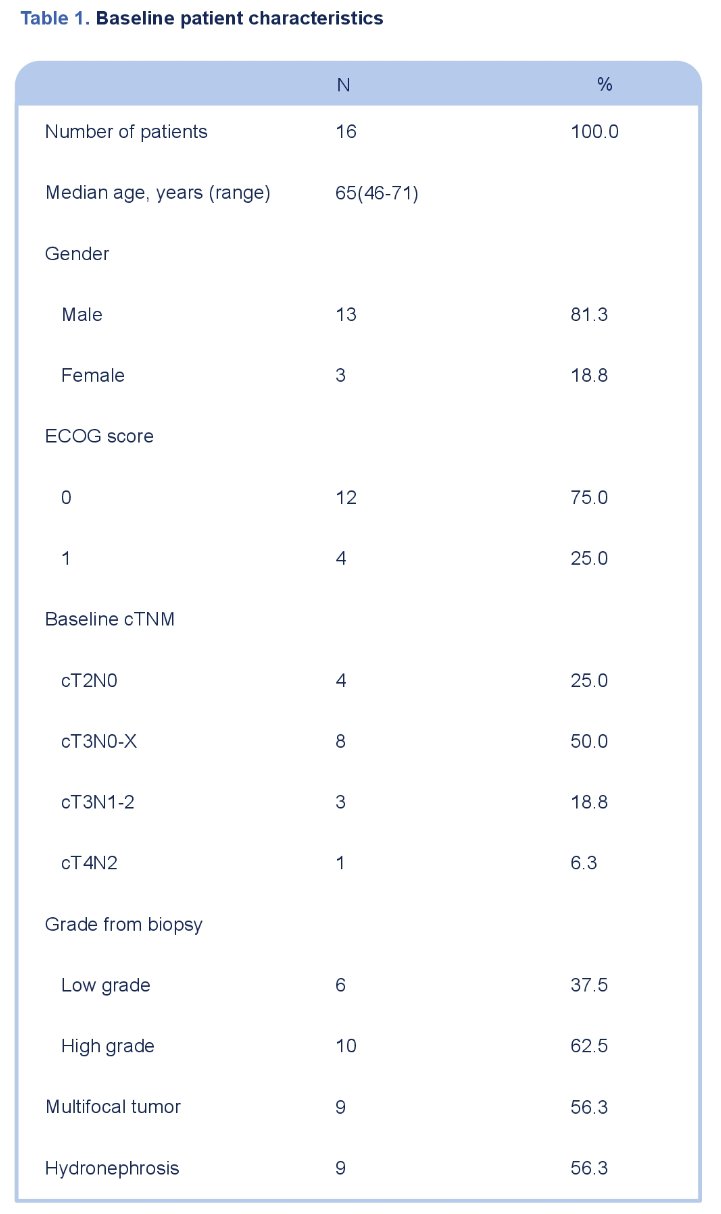
All patients received ≥2 doses of tislelizumab, but 7/16 patients did not receive all four doses as planned. Among the 16 patients, 13 underwent surgery (radical nephroureterectomy: 9, segmental ureteral resection: 3, endoscopic ablation: 1). Three patients declined surgery due to disease progression or adverse events. The median follow-up was 25 months. The best radiological tumor response prior to surgery was:
- Complete response: 0
- Partial response: 38%
- Stable disease: 44%
- Progressive disease: 19%

A pathologic complete response (ypT0) was noted in 4/16 patients, of whom 3 underwent a radical nephroureterectomy and 1 endoscopic ablation. 7/16 patients had ≤ypT1N0 disease.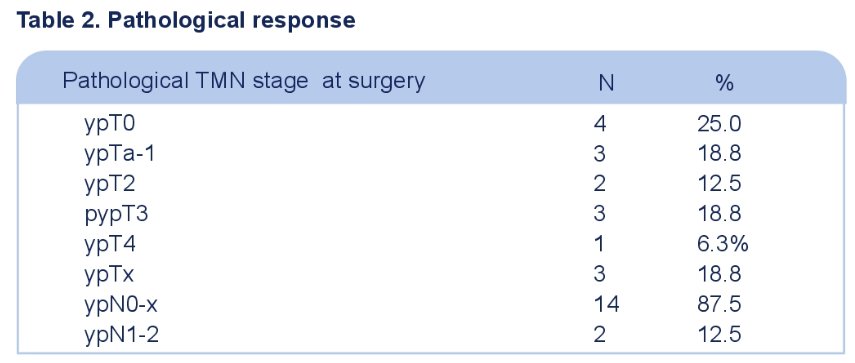
The median overall and progression-free survivals have not been reached yet.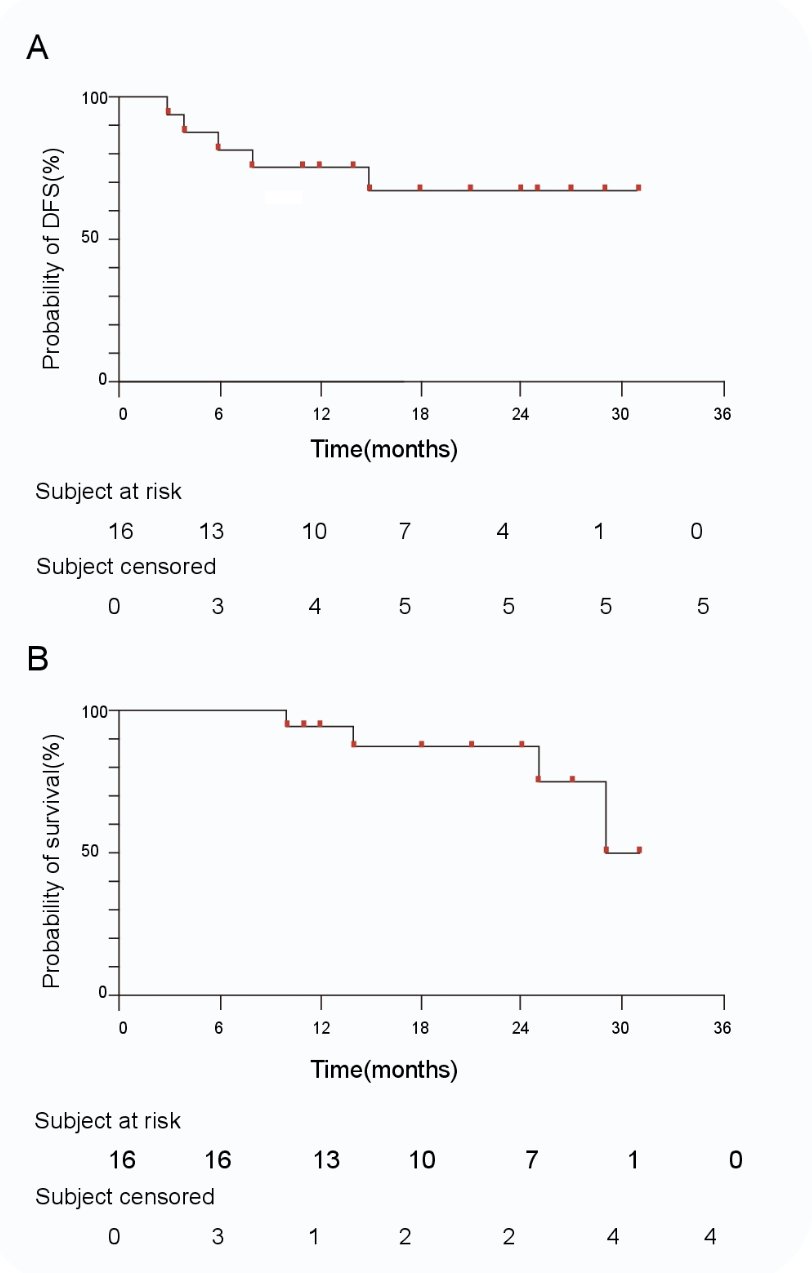
Grade 3-4 TRAEs occurred in 19% of patients. There were no new safety signals observed. 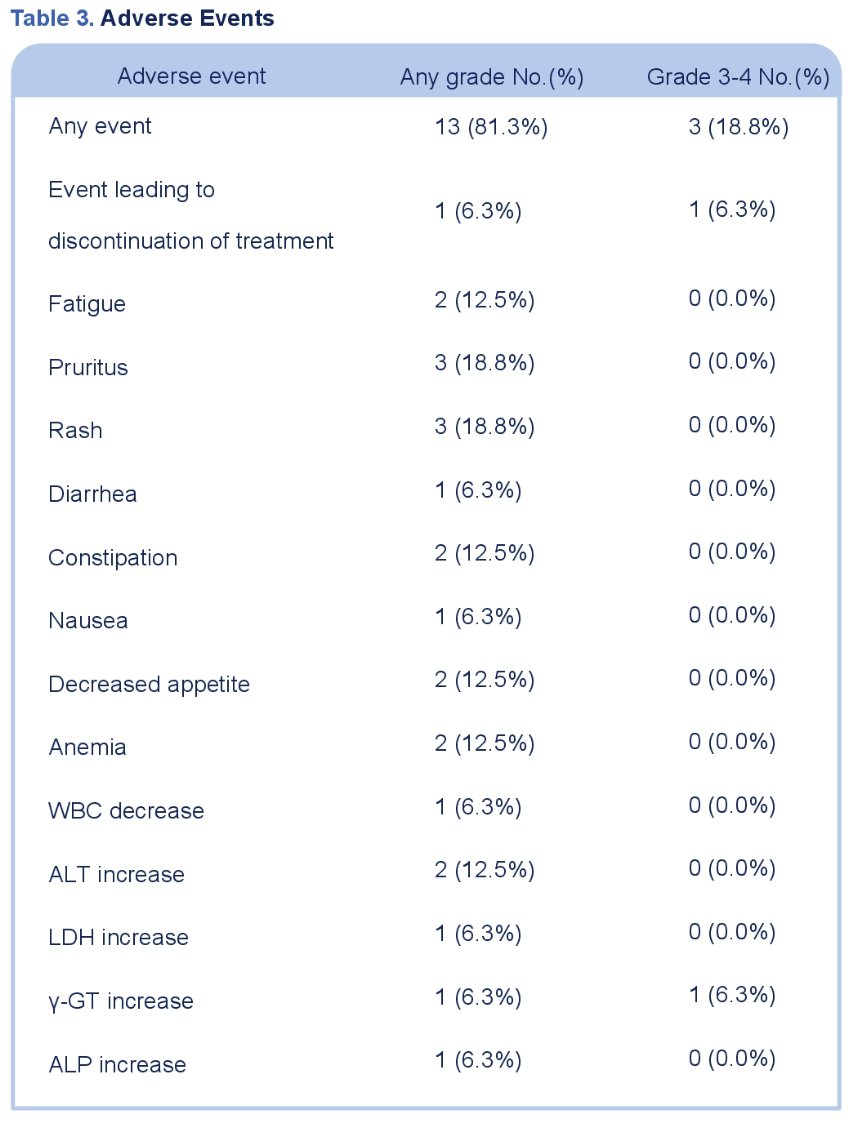
Dr. Huang concluded that this trial of neoadjuvant tislelizumab in patients with cisplatin-ineligible, high-risk UTUC demonstrates promising pathologic complete response rates. Further exploratory analysis is ongoing to investigate the underlying correlation between tumor immune microenvironment and treatment response.
Presented by: Jiwei Huang, BS, Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet 2020 Apr 18;395(10232):1268-1277.
- Chen H, Yang W, Xue X, et al. Neoadjuvant immunotherapy and chemoimmunotherapy for stage II-III muscle invasive bladder cancer. Front Immunol. 2022;13:986359.


