(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma trials in progress poster session. Dr. Robert Svatek presented the PIVOT-006 trial, a phase 3, randomized study of cretostimogene grenadenorepvec (CG) versus observation for the treatment of intermediate risk non-muscle invasive bladder cancer (NMIBC) following transurethral resection of bladder tumor (TURBT).
Cretostimogene is a conditionally replicating, intravesically delivered adenovirus that induces oncolytic immunotherapy. Cretostimogene is engineered to selectively replicate and lyse Rb-altered bladder cancer cells via insertion of human E2F-1 promoter and producing GM-CSF, stimulating the immune system via a dual mode of action.
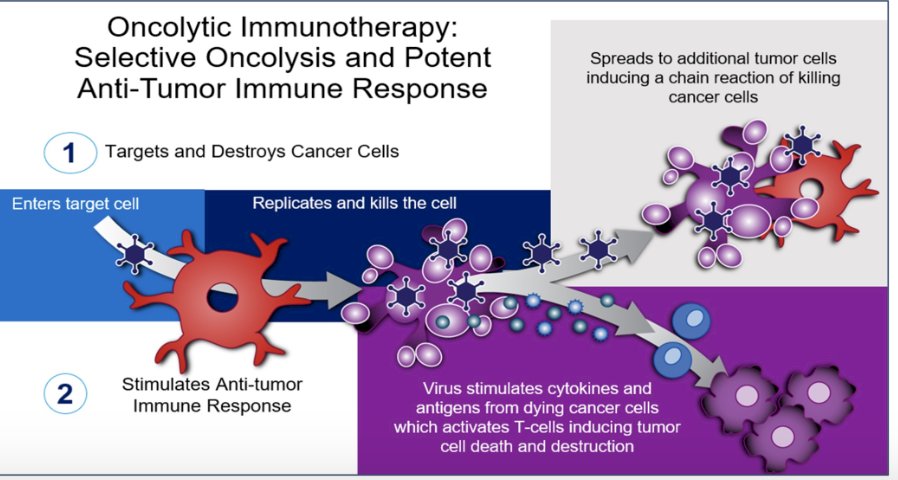
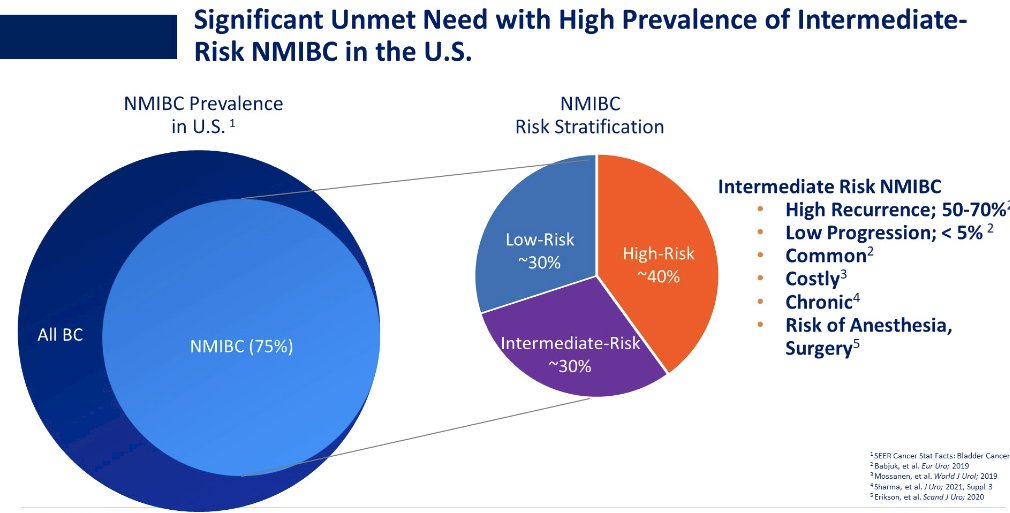
In high-risk NMIBC, cretostimogene shows complete response rates between 46 and 85%.1,2 In intermediate-risk NMIBC, current guidelines recommend intravesical therapy or observation, yet recurrence rates remain high at 30 to 60%.
PIVOT-006 is an open label phase 3, randomized study of cretostimogene grenadenorepvec versus observation for the treatment of intermediate-risk NMIBC following TURBT, using the AUA criteria.
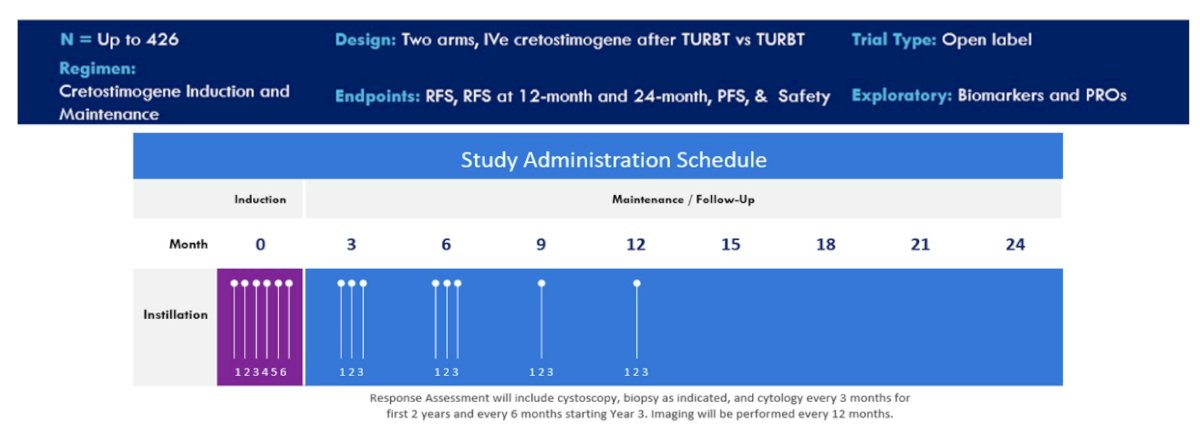
Patients will undergo 1:1 randomization, stratified by receipt of perioperative chemotherapy and tumor grade, with an option to crossover from the observation to the control arm if recurrence occurs in the observation arm. The target sample size is 426 patients.
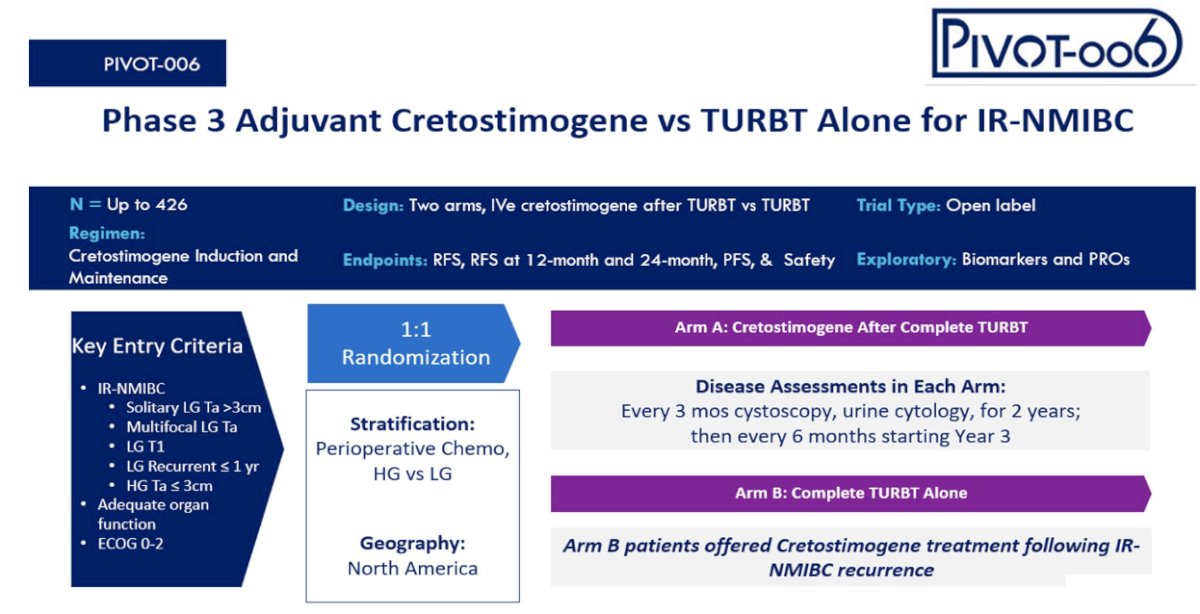
The primary outcome is recurrence-free survival. Disease assessment will be performed with cystoscopy and cytology every 3 months for the first two years, followed by every 6 months starting year 3.The investigators will also assess patient-reported outcomes and biomarker analysis is planned as well.
Presented by: Robert S. Svatek, MD, Professor and Chair, Gary and Glenda Woods President’s Distinguished University Chair in GU Oncology, Department of Urology, UT Health San Antonio, TX
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related content: PIVOT-006 - A Study of Intravesical Cretostimogene Grenadenorepvec for Treatment of Patients with Intermediate-Risk, Non-Muscle Invasive Bladder Cancer - Mark Tyson
References:
1. Burke JM, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012;188(6):2391-7.
2. Packiam VT, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. 2018;36(10):440-7.
3. Messing EM, Tangen CM, Lerner SP, et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA. 2018;319(18):1880-8.


