(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma poster session. Dr. Daniel Castellano presented the results of BladderGATE, a phase I-II ONCOSUR study of atezolizumab plus intravesical Bacillus Calmette-Guerin (BCG) upfront combination in patients with high risk, non-muscle invasive bladder cancer (NMIBC).
Dr. Castellano noted that adjuvant intravesical BCG induction + maintenance following TURBT is the current standard of care for patients with high-risk NMIBC. However, recurrence rates remain high at 30-40% and 70-80% at two and five years, respectively.1 Atezolizumab is an anti-PD-L1 IgG1 monoclonal antibody that is associated with long-term durable remissions in patients with metastatic urothelial carcinoma2 and BCG-unresponsive patients.3 Dr. Castellano and colleagues hypothesized that atezolizumab in combination with standard BCG could provide synergistic benefit for patients with NMIBC. BladderGATE (NCT04134000) is a phase 1b/2 study evaluating the safety and efficacy of upfront atezolizumab + intravesical BCG in high-risk NMIBC patients.
In this phase 1b/2 open label clinical trial, patients were scheduled to receive atezolizumab 1,200 mg IV on day 1 of each 21-day cycle (maximum of 52 weeks) plus BCG weekly x 6 weeks induction followed by maintenance at weeks 12, 24, and 48.
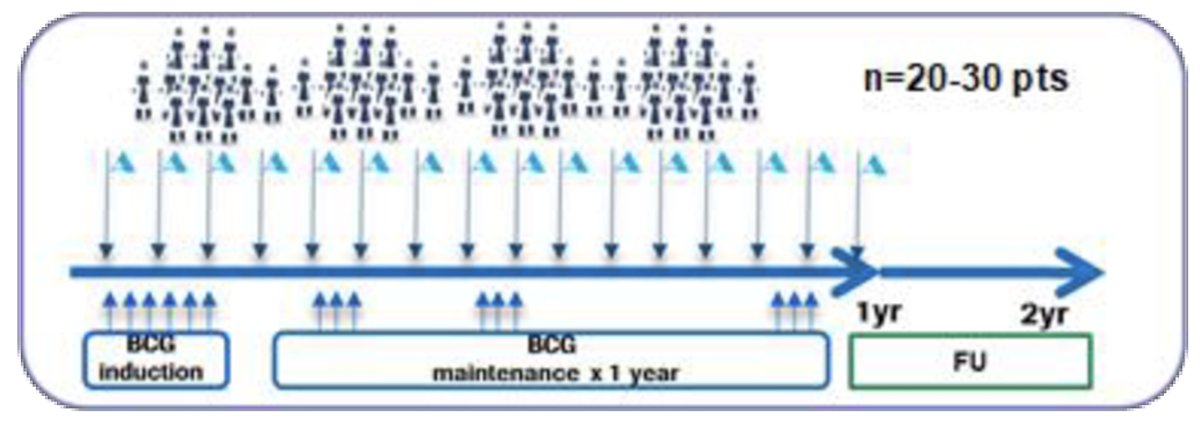
This study included patients with high-risk NMIBC (i.e., T1, HG Ta, and/or CIS) and were either BCG-naïve or stopped BCG ≥2 years ago, without history of prior radiation to the bladder.

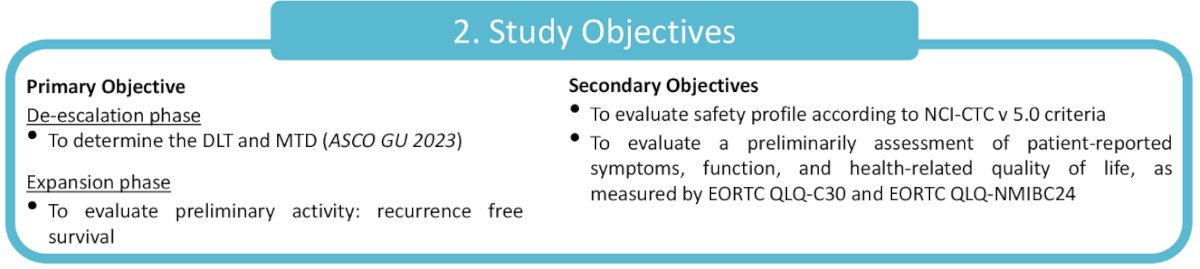
The study objectives are as below:
Baseline characteristics are summarized below. The median patient age was 70 years. 44% of patients had evidence of multifocal disease, with the same proportion having tumors ≥3 cm in size.

In this trial, 56% of patients completed all BCG instillation, with 89% overall considered to have received ‘adequate’ BCG per the FDA guidance. Atezolizumab treatment was completed by 61% of patients, with a median of 14.5 doses. The main reason for atezolizumab non-compliance was immune-related adverse events, with grade ≥3 such events occurring in 19.4% of patients.

With regards to efficacy outcomes, local recurrence occurred in 17% of patients (13.9% high grade), with a 2-year high-grade disease-free survival of 73%. Three (8%) patients progressed to muscle invasiveness.
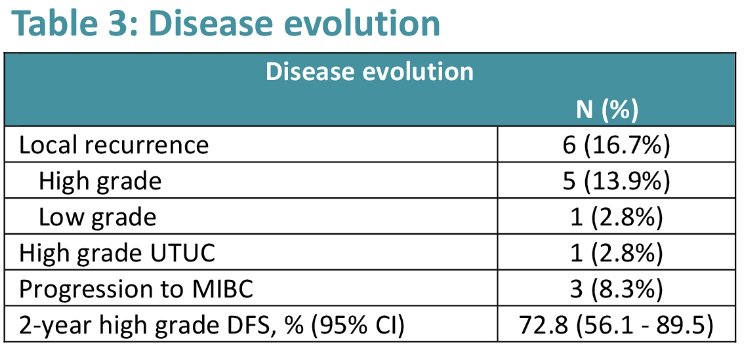
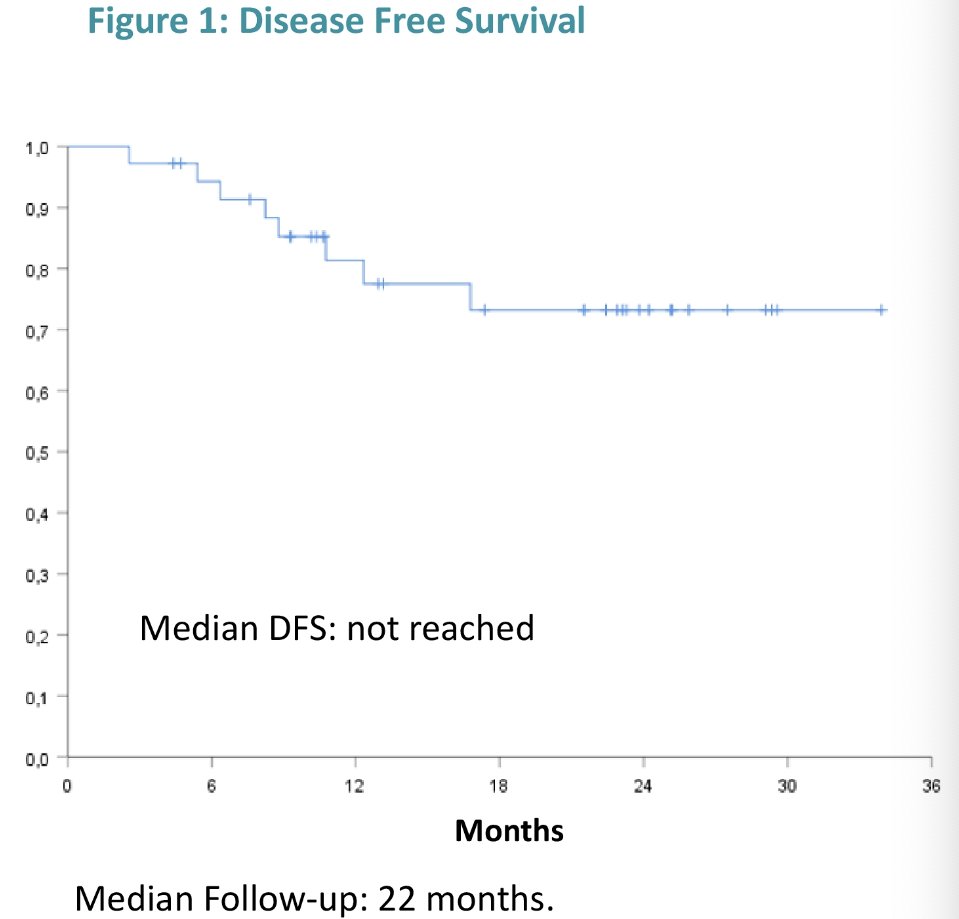
Dr. Castellanos concluded that the combination strategy of upfront atezolizumab + intravesical BCG for high-risk NMIBC patients appears feasible and safe. The 2-year local recurrence rate with such an approach is 14% and only 8% of patients had evidence of progression to muscle invasiveness.
Presented by: Daniel Castellano, MD, Head, Medical Oncology Department, University Hospital 12 de Octubre, Madrid, Spain
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
1. Gontero P, et al. Guidelines on Non-muscle-invasive bladder cancer (Ta, T1 and CIS). In: EAU Guidelines published at the 38th Annual Congress Milan 2023. European Associaton of Urology Guidelines Office, Arnhem, the Netherlands.


