(UroToday.com) The 2024 GU ASCO annual meeting included a urothelial carcinoma session featuring trials in progress and a presentation by Dr. Andrea Necchi discussing the trial design of GDFather-Neo, a neoadjuvant immunotherapy trial of visugromab (CTL-002) in combination with the anti-PD1 antibody nivolumab. Neoadjuvant chemotherapy is a well-established treatment modality in MIBC but suffers from limited activity and significant toxicity. Recently, neoadjuvant immunotherapy demonstrated clinical safety and efficacy in various solid tumors including MIBC, with potentially less toxicity. Results from previous studies of single-agent immunotherapy indicated a proportion of pathologic complete responses (ypT0N0) similar to that reported with neoadjuvant chemotherapy. Therefore, improving ypT0N0 responses remains a major task for treatment of MIBC.
Increasing evidence has emerged that Growth and Differentiation Factor 15 (GDF-15) plays a critical local immunosuppressive role. Apart from blocking immune-cell entry into tissues, GDF-15 also has major impact on the formation of the immune synapse. Many tumors overexpress GDF-15 and have hijacked this mechanism to block immunotherapy therapy success. Various translational research efforts indicate that GDF-15 may play a significant role for immunosuppression and T-cell exclusion in urothelial carcinoma.
Visugromab (CTL-002) is a GDF-15 neutralizing IgG4 monoclonal antibody that demonstrated in phase 1 a favorable safety profile and promising clinical activity with durable and deep responses in PD-1/PD-L1 relapsed/refractory metastatic solid tumors in combination with the anti-PD1 antibody nivolumab. The Neo-GDFather trial is intended to investigate the combination of visugromab with nivolumab versus nivolumab monotherapy as neoadjuvant therapy for MIBC in patients who are ineligible for or elect not to undergo neoadjuvant chemotherapy.
This is a multi-center, parallel-cohort, single-blinded phase 2 study of neoadjuvant therapy in patients planned for radical cystectomy A total of 30 subjects with stage T2-T4N0M0 MIBC will be enrolled and assigned 1:1 to receive either nivolumab + visugromab or nivolumab + placebo after stratification for CPS PD-L1 expression and cT-stage. The trial design for GDFather-Neo is as follows:
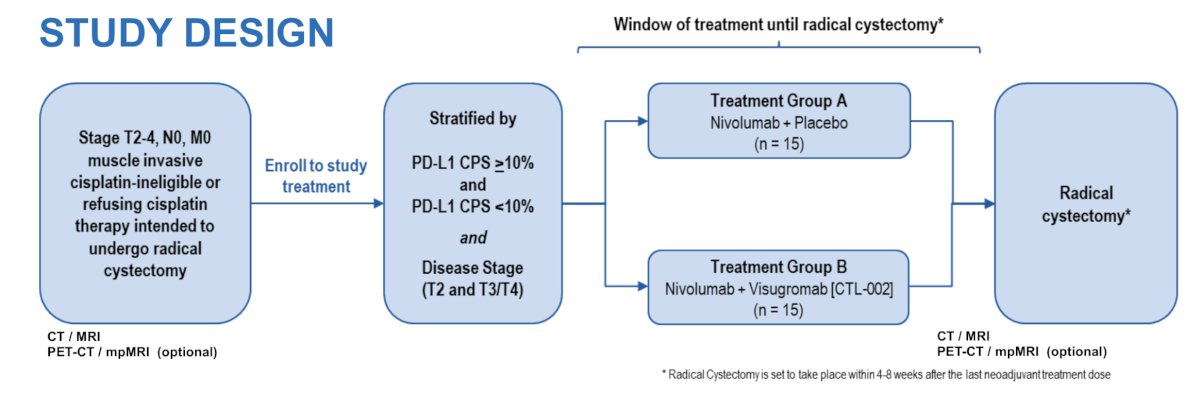
Other inclusion criteria comprise an ECOG performance status 0-1 and a pure/predominant urothelial carcinoma histology. No statistical assumptions were undertaken at this stage. Treatment consists of three 4-week cycles and radical cystectomy is planned 4-8 weeks after last dose of study drug. After radical cystectomy, patients will follow standard recommendations of EAU guidelines. Primary endpoints are the proportion of ypT0N0 response and radiologic response rate. Secondary endpoints comprise additional efficacy parameter, surgical and medical safety, PK and PD assessments. Translational research includes evaluation of immunologic parameters in the tumor, other immune-correlates and molecular profiles, as well as evaluation of treatment-emergent cytokine and chemokine profiles in peripheral blood.
Dr. Necchi concluded his presentation highlighting the GDFather-Neo trial with the following concluding messages:
- Neutralization of GDF-15 is a promising approach to overcome tumor immune escape and immunosuppression
- The clinical stage anti-GDF-15 antibody visugromab (CTL-002) has demonstrated a favorable safety profile and impressive clinical activity in combination with anti-PD1 in PD1/PD-L1 refractory/relapsed metastatic solid tumors
- The GDFather-Neo Study is intended to demonstrate an increase of the pathologic complete response rate in combination with nivolumab in patients with MIBC compared to nivolumab treatment alone
Presented by: Andrea Necchi, MD, Vita-Salute San Raffaele University, IRCCS San Raffaele Hospital, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


