(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a urothelial carcinoma oral abstract session. Dr. Michiel van der Heijden presented subgroup analyses from EV-302, a phase 3 global trial of enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated locally advanced metastatic urothelial carcinoma.
There have been few advances addressing the high unmet need in advanced urothelial cancer, with the median overall survival hovering around 13 months over the last few decades.1 Given the reliance on cisplatin-based combination chemotherapy in the front-line setting, cisplatin-eligibility has long defined 1st line treatment for locally advanced and metastatic urothelial carcinoma. PD-1/L1 inhibitors are available as maintenance therapy for a subset of patients or as second line therapy.2,3 And more recently, the addition of nivolumab to cisplatin-gemcitabine has improved overall survival in the cisplatin-eligible population.4
Recently, the combination of enfortumab vedotin plus pembrolizumab has been approved by the FDA for the treatment of locally advanced/metastatic urothelial carcinoma based on a favorable benefit/risk versus platinum-based chemotherapy in EV-302/KEYNOTE-A39 (NCT04223856), which was presented at ESMO 2023. In this report, Dr. Dr. van der Heijden presented the results for the pre-specified subgroup analyses from EV-302.
The study design is illustrated below. This trial included previously untreated patients with locally advanced/metastatic disease who were platinum eligible (eGFR≥30) and PD-(L)1 inhibitor naïve. These patients were randomized to enfortumab vedotin + pembrolizumab versus chemotherapy (cisplatin/carboplatin + gemcitabine). The dual primary endpoints were progression-free survival, assessed via blinded independent central review, and overall survival.

Compared to platinum-based chemotherapy, the combination of enfortumab vedotin + pembrolizumab prolonged progression-free survival from 6.3 to 12.5 months (HR: 0.45, 95% CI: 0.38 – 0.54, p<0.00001), and the overall survival from 16.1 to 31.5 months (HR: 0.47, 95% CI: 0.38 – 0.58, p<0.00001).

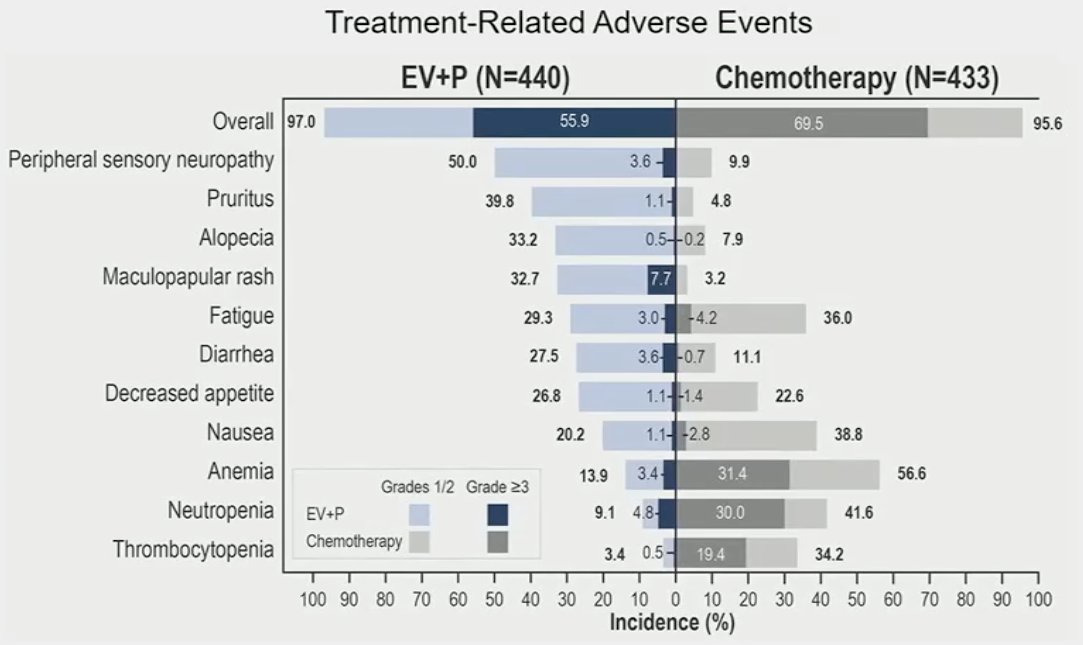
With regards to safety outcomes, the combination of enfortumab vedotin + pembrolizumab was generally manageable with no new safety signals observed. Grade ≥3 events were observed in 56% of patients in the experimental arm, compared to 70% in the chemotherapy arm. Investigator-assessed, treatment-related adverse events leading to death occurred in 4 (0.9%) patients in the enfortumab vedotin + pembrolizumab arm (asthenia, diarrhea, immune-mediated lung disease, multiple organ dysfunction syndrome).
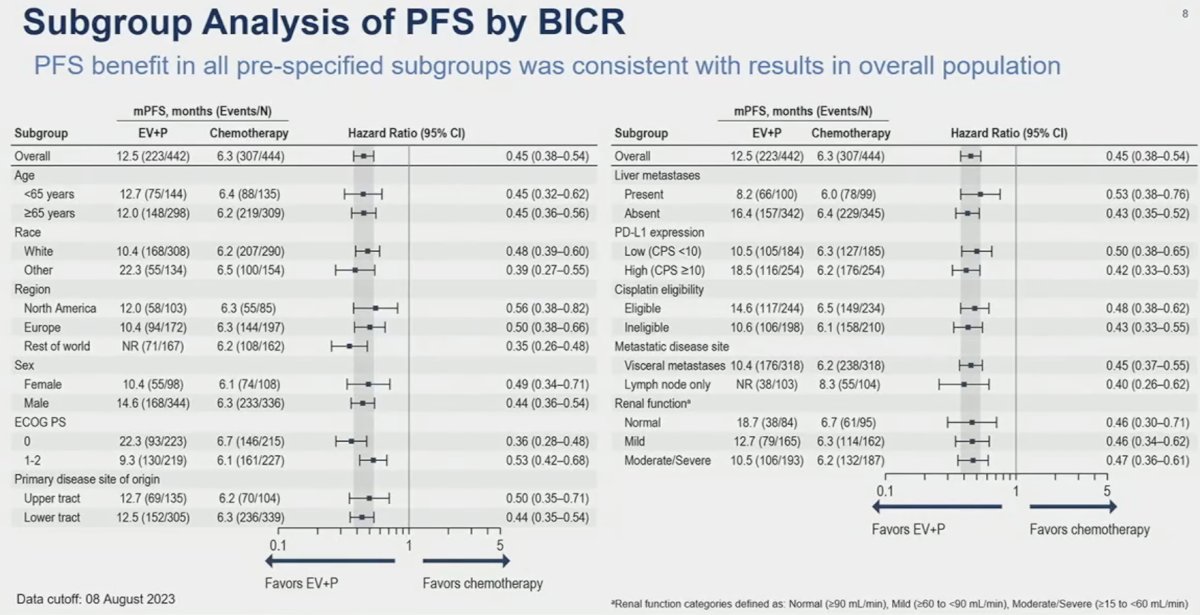
On subgroup analyses, consistent PFS results were observed in favor of enfortumab vedotin + pembrolizumab, irrespective of cisplatin eligibility (eligible versus ineligible) and PD-L1 status (high versus low), with HRs consistently in the 0.42 – 0.50 range.

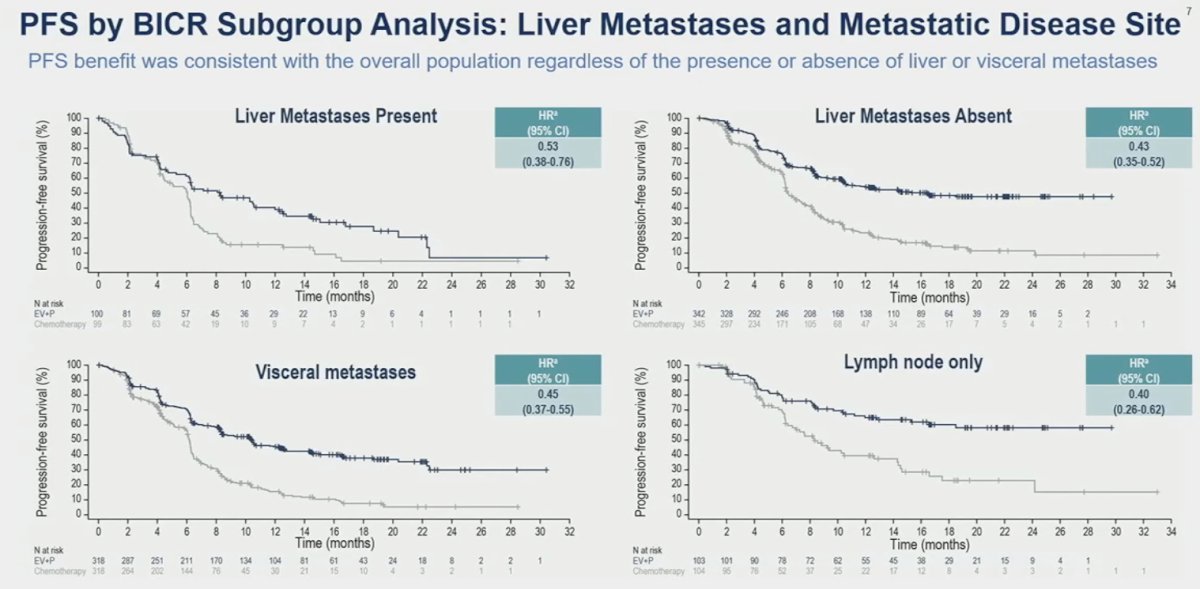
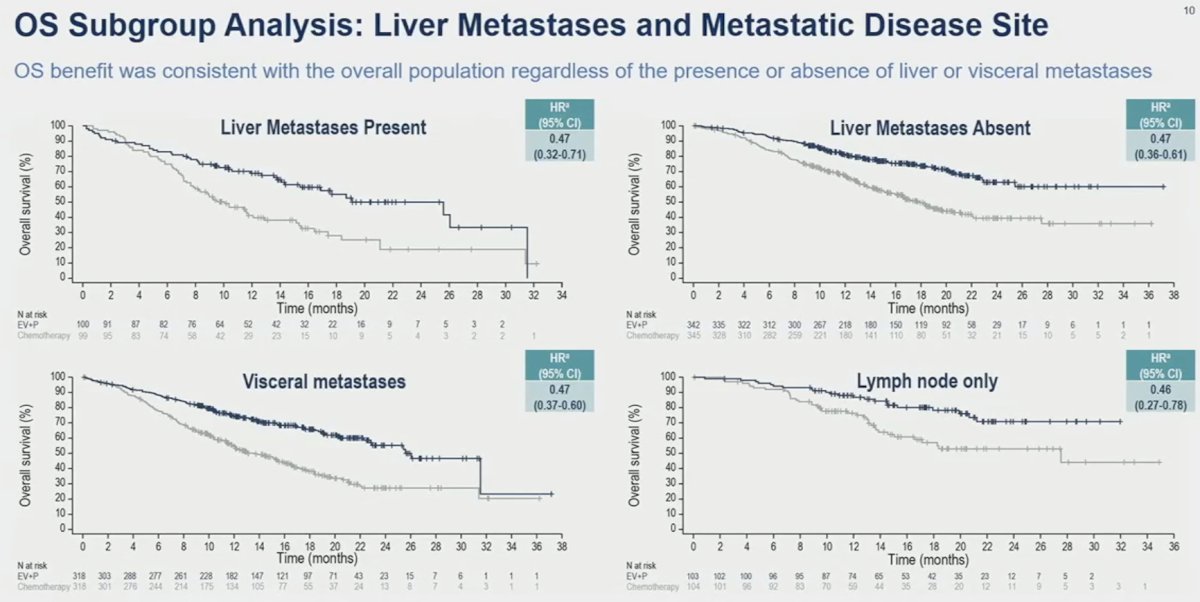
When stratified by site/extent of metastatic disease spread, enfortumab vedotin + pembrolizumab consistently outperformed chemotherapy irrespective of presence/absence of liver metastases and presence of visceral versus lymph node only metastases.
Additional PFS subgroup analyses by age, race, geographic region, sex, performance status, primary disease site origin (upper versus lower tract), and renal function all consistently favored enfortumab vedotin + pembrolizumab.
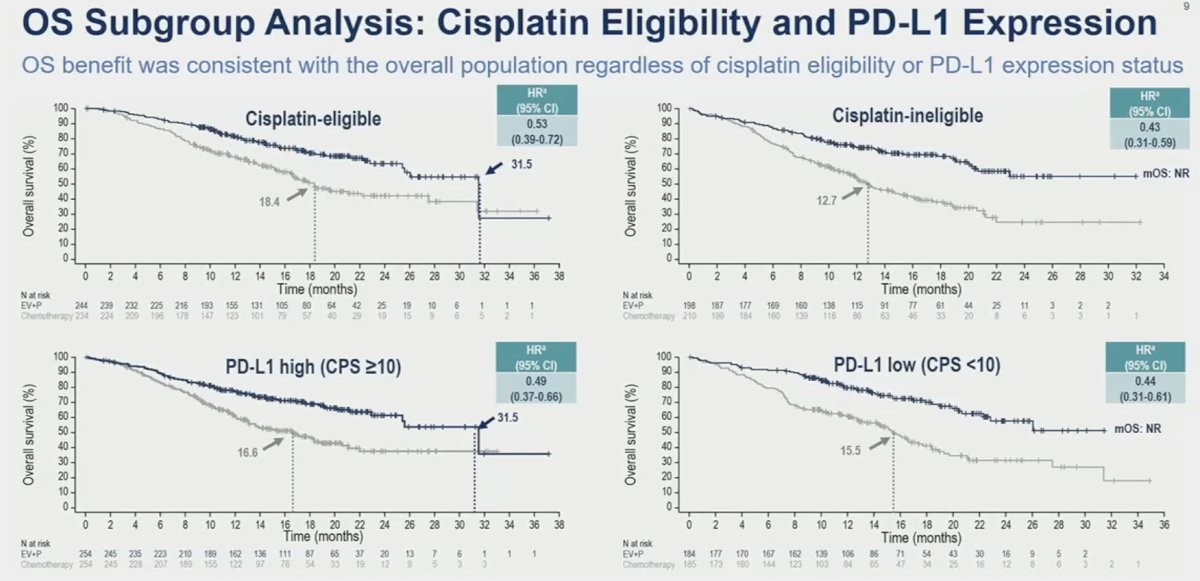
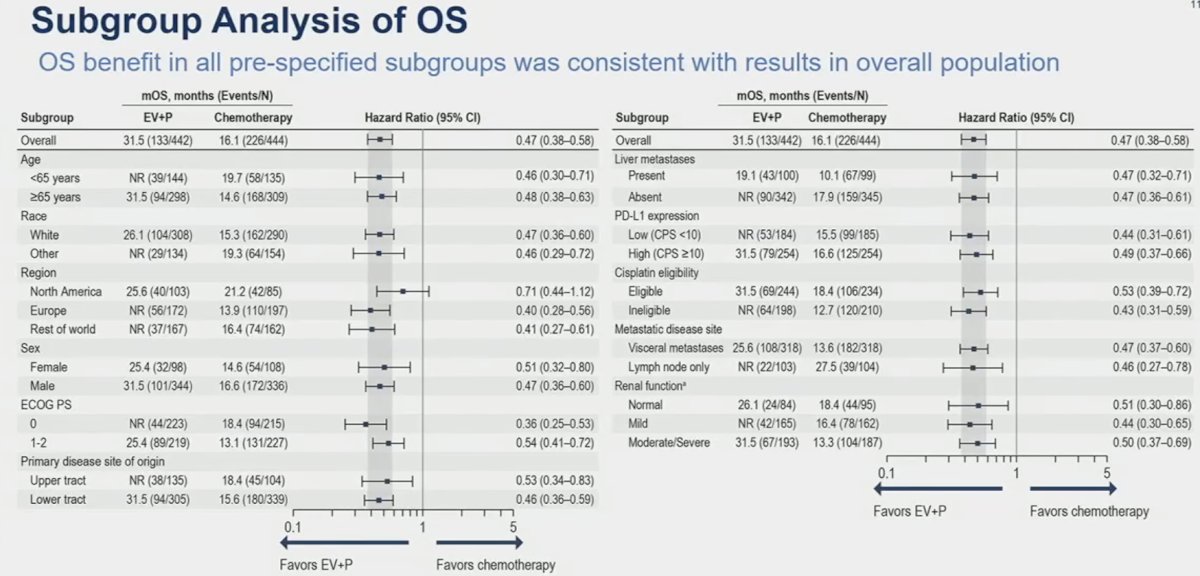
Similar results were consistently observed for the overall survival subgroup analyses in favor of enfortumab vedotin + pembrolizumab, with clinically meaningful HR and median survival improvements across all subgroup comparisons.
When objective response rate subgroup analyses were performed, a similarly consistent pattern was observed across all evaluable subgroups:

Dr. van der Heijden concluded that:
- Enfortumab vedotin + pembrolizumab significantly improved progression-free and overall survivals, compared to platinum-based chemotherapy, in a broad patient population in the EV-302/KEYNOTE-A39 trial
- Median progression-free and overall survivals are nearly doubled in the enfortumab vedotin + pembrolizumab arm
- Based on these results, the FDA has granted enfortumab vedotin + pembrolizumab approval for the treatment of locally advanced/metastatic urothelial carcinoma
- The magnitude of benefit observed with enfortumab vedotin + pembrolizumab was similar between cisplatin-eligible and cisplatin-ineligible subgroups
- Enfortumab vedotin + pembrolizumab benefit in all pre-specified subgroups was consistent with the overall patient population
- These results further support enfortumab vedotin + pembrolizumab as a new standard of care in locally advanced/metastatic urothelial carcinoma
Presented by: Michiel Simon van der Heijden, MD, PhD, Department of Medical Oncology, Netherlands Cancer Institute, Amsterdam, The Netherlands
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related content: EV-302: Enfortumab Vedotin + Pembrolizumab Outperforms Chemo in Untreated Advanced Urothelial Cancer - Michiel Simon Van der Heijden
References:
- Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012 Apr 1;30(10):1107-1113.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After >/=2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.
- van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023;389(19):1778-1789.


