(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma oral abstract session. Dr. Rohit Jain presented the results of PemCab, a non-randomized phase II study of cabozantinib plus pembrolizumab as first-line therapy for cisplatin-ineligible advanced urothelial carcinoma.
Treatment options for cisplatin-ineligible and, especially, platinum-ineligible patients with locally advanced/metastatic urothelial carcinoma are limited. Avelumab maintenance is only available to patients who do not progress after platinum-based chemotherapy.1 Pembrolizumab monotherapy is available to only select platinum-ineligible patients.2 More recently, the combination of enfortumab vedotin + pembrolizumab was approved in December 2023 as 1st line therapy for all locally advanced/metastatic urothelial carcinoma patients.
Vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) have demonstrated activity as monotherapy in pre-treated metastatic urothelial carcinoma. Cabozantinib is a multiple receptor TKI targeting MET, VEGFR-2, RET, KIT, TIE-2, and the TAM family of kinases (TYRO2, AXL, and MER). Preclinical and clinical studies have demonstrated that cabozantinib combined with PD-1 inhibitors has encouraging safety and efficacy results. In his presentation, Dr. Jain presented data from a phase II trial reporting the activity and safety of 1st line combination of cabozantinib and pembrolizumab in (cisplatin-ineligible/PD-L1 positive) and cisplatin-refusing or platinum ineligible locally advanced/metastatic urothelial carcinoma.
The PemCab study design is summarized below. Eligible patients were administered pembrolizumab 200 mg IV every 3 weeks plus cabozantinib 40 mg orally once daily. The primary endpoint was objective response rate, per RECIST v1.1. Secondary endpoints included:
- Progression-free survival
- Overall survival
- Duration of response
- Disease control rate
- Safety

The target sample size was 39 patients. The statistical plan is summarized below:

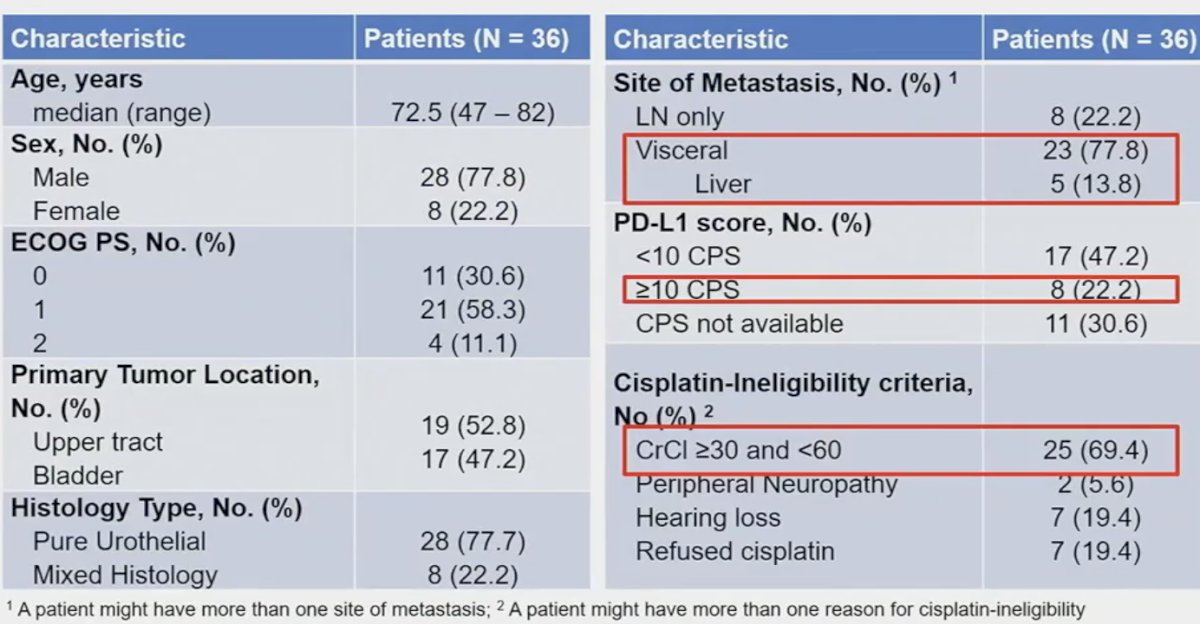
The study cohort included 36 patients, enrolled between December 2018 and April 2023. The baseline characteristics are summarized below. The median participant age was 72.5 years. Over half (53%) patients had primary upper tract disease, and 78% had pure urothelial histology. 78% of patients had visceral metastases (liver: 14%). 22% of patients were PD-L1 positive. The most common reason for cisplatin-ineligibility was a creatinine clearance between 30 and 60 m:/min (69%).
At data cutoff, only one patient (2.7%) remained on treatment. 50% of patients discontinued treatment due to disease progression, and a further 22% went off treatment due to adverse events.

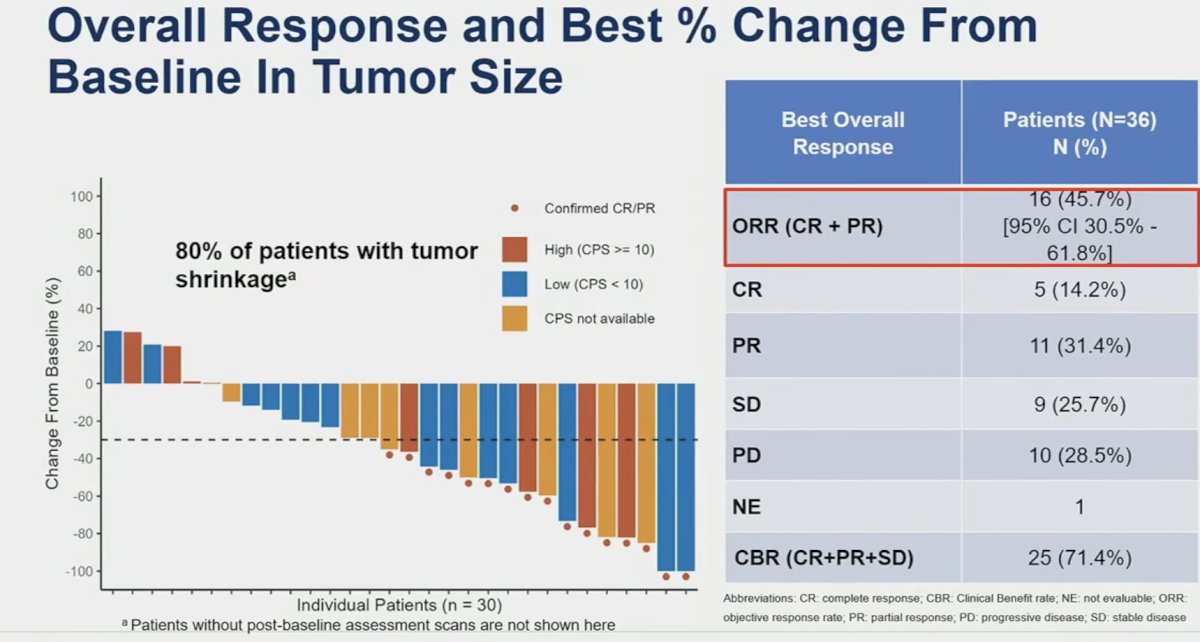
From an efficacy standpoint, an ORR was observed in 16 patients (46%), of whom 4 (14%) had a complete response. A further nine patients had stable disease, for a clinical benefit rate of 71.4%. On radiographic evaluation, 80% of patients demonstrated evidence of tumor shrinkage.
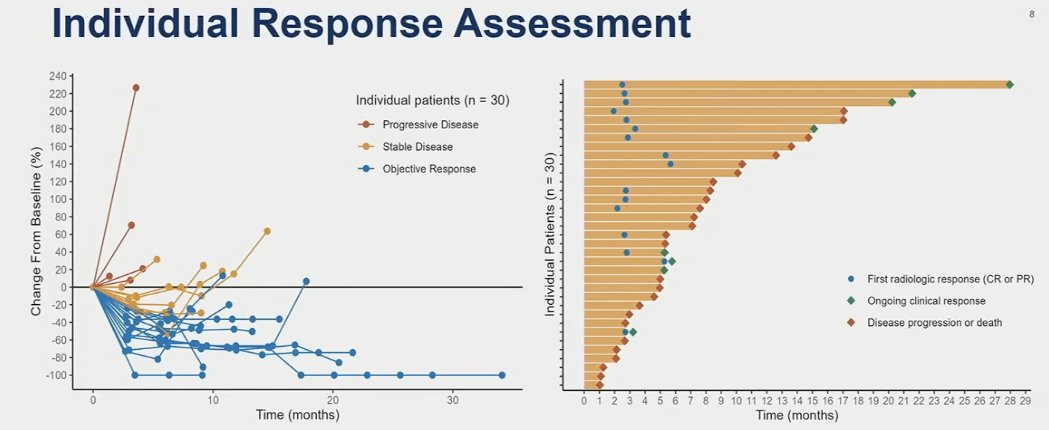
The median time to response was 2.7 months, and the median duration of response was 14.7 months. Eight patients had evidence of ongoing clinical response as of their last clinic follow-up.
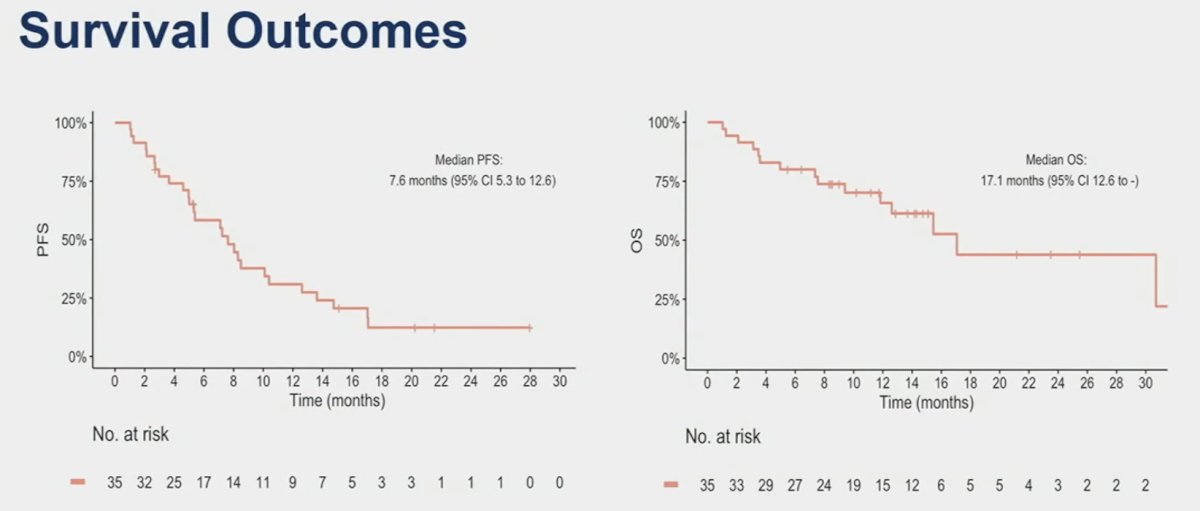
At a median follow-up of 14.3 months, the median progression-free and overall survivals were 7.6 and 17.1 months, respectively.
At least one treatment-emergent adverse event (TEAE) was observed in 92% of patients. Grade ≥3 TEAEs were observed in 50% of patients. Two grade 4 treatment-related adverse events related to cabozantinib occurred, which included gastrointestinal bleeding and colonic perforation. No treatment-related deaths occurred. 83% of patients experienced immune-related events, and 11% required high dose steroid due to such events. The median number of cycles received were:
- Pembrolizumab: 10 cycles
- Cabozantinib: 7.5 cycles
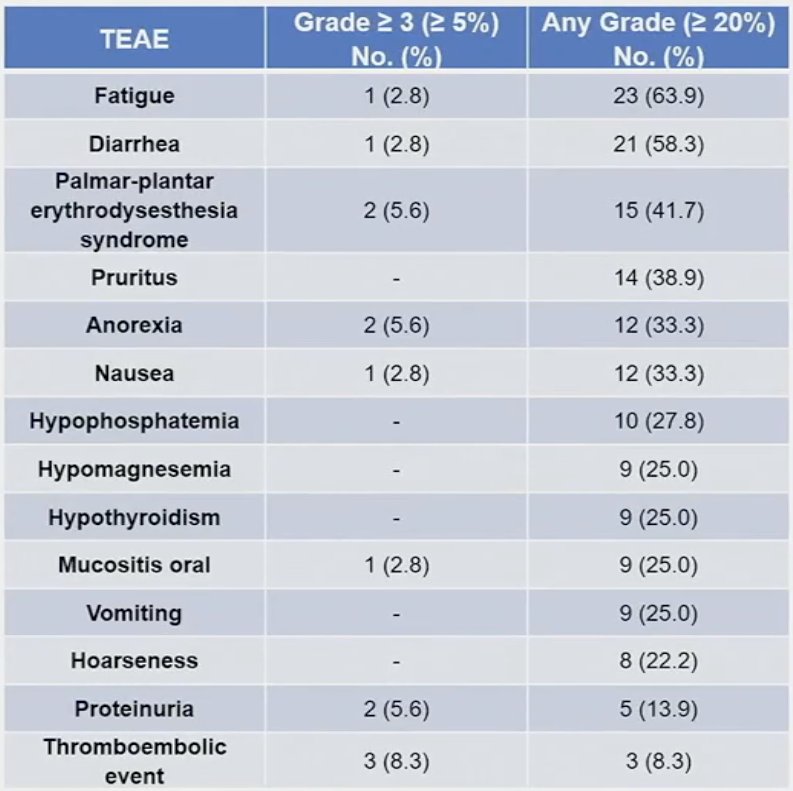
Dr. Jain concluded as follows:
- In the cisplatin-ineligible PD-L1 positive and cisplatin-refusing/platinum-ineligible population, the combination of cabozantinib + pembrolizumab showed encouraging efficacy:
- ORR: 46%
- Disease control rate: 71%
- PFS, median: 7.6 months
- OS, median: 17.1 months
- This trial did not attain the 17 responses required to exclude a lower bound of 95% CI ORR=32%
- Cabozantinib + pembrolizumab displayed a manageable safety profile with no new safety signals
- 1st line lenvatinib + pembrolizumab did not improve outcomes in the LEAP-011 phase 3 trial; MAINCAV is evaluating the combination of cabozantinib with maintenance avelumab
- Further evaluation of cabozantinib + pembrolizumab may be warranted, informed by principles of precision medicine (biomarker studies are ongoing). Potentially, a more tolerable and potent VEGFR TKI similar to cabozantinib (e.g., zanzalintinib) may be an important direction of investigation to improve the therapeutic index
Presented by: Rohit K. Jain, MD, MPH, Assistant Member, Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:1. Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.


