(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma oral abstract session. Dr. Andrea Apolo presented the late-breaking analysis of AMBASSADOR (Alliance A031501), a phase 3 randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma versus observation.
Muscle-invasive urothelial carcinoma is an aggressive disease with high relapse rates. In addition to trimodality therapy in select patients, the current standard of care is radical cystectomy +/- neoadjuvant chemotherapy. However, many patients are cisplatin-ineligible or have persistent muscle-invasive disease following neoadjuvant chemotherapy and surgery. Adjuvant cisplatin-based therapy is currently not recommended for patients who have received neoadjuvant chemotherapy. However, it does improve disease-free survival in patients not previously treated with neoadjuvant chemotherapy, at the cost of increased toxicity, and may be difficult to initiate post-radical surgery.
Pembrolizumab is a Programmed Death protein (PD-1) checkpoint inhibitor that is approved as monotherapy and in combination with enfortumab vedotin for the treatment of locally advanced/metastatic urothelial carcinoma and for BCG-unresponsive, high-risk NMIBC. In this presentation. Dr. Apolo reported the interim results of the open-label, phase 3 ALLIANCE AMBASSADOR trial comparing pembrolizumab to observation as adjuvant therapy in patients with high-risk, muscle-invasive urothelial carcinoma
The study design is summarized below. Eligible patients were those with muscle-invasive urothelial carcinoma of the lower or upper tracts who were 4 – 16 weeks post-radical surgery (cystectomy, nephrectomy, nephroureterectomy, ureteral resection) with the following pathologic findings:
- Post-neoadjuvant chemo: ≥ypT and/or ypN+/positive margins
- Cisplatin-ineligible or refusing: ≥pT3 and/or pN+/positive margins
Patients underwent stratified 1:1 randomization to pembrolizumab 200 mg IV every 3 weeks for 1 year (18 cycles) versus observation. The dual primary endpoints were disease-free and overall survival. The key secondary endpoints were survival outcomes by PD-L1 status and safety outcomes.

The statistical design is summarized below. The target sample size was 739 patients, and the trial would be considered ‘positive’ if either the disease-free survival (alpha<0.01) or overall survival (alpha <0.005) endpoints were met. In this report, Dr. Apolo presented the second interim results of the trial.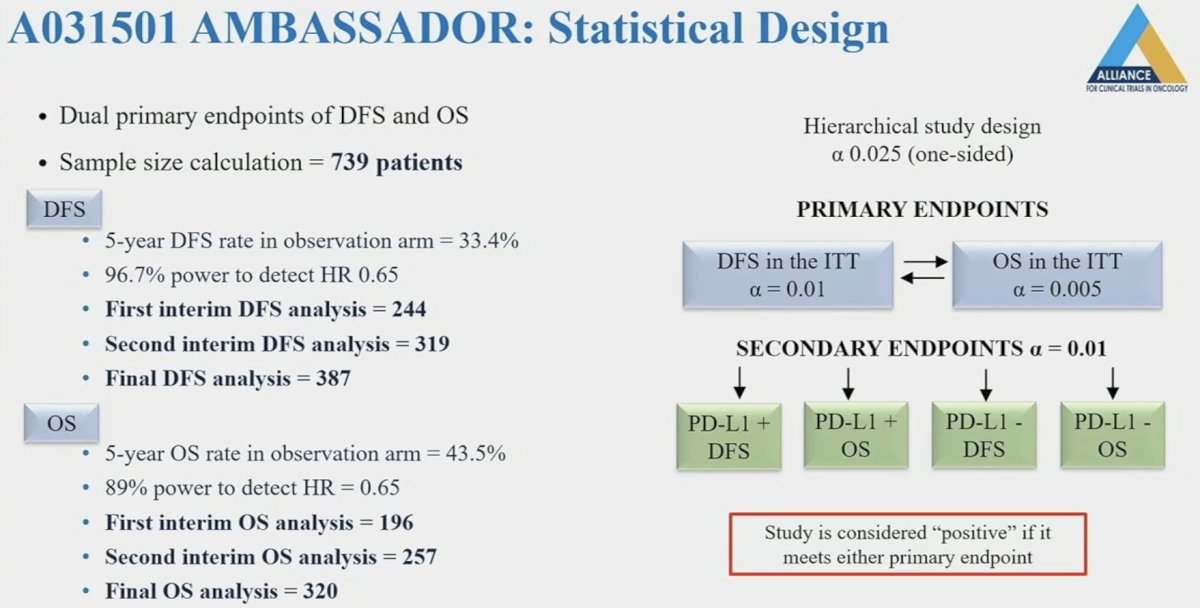
702 of the planned 734 patients were enrolled between September 2017 and August 2021. The trial was closed early due to the FDA approval of adjuvant nivolumab for muscle invasive urothelial carcinoma. With regards to DFS, such events occurred in 41.5% and 49.4% of patients in the pembrolizumab and observation arms, respectively. Overall survival events occurred in 37% and 36.2% of participants, respectively.
The baseline patient characteristics are summarized below. Approximately 2/3 of patients received neoadjuvant therapy. While a positive surgical margin status was a ‘unique’ eligibility criteria in this trial, only 2.4% of patients were enrolled due to a positive margin status. Almost 60% of patients were PD-L1+. Approximately 22% of patients had upper tract disease (enrolment of these patients was not capped).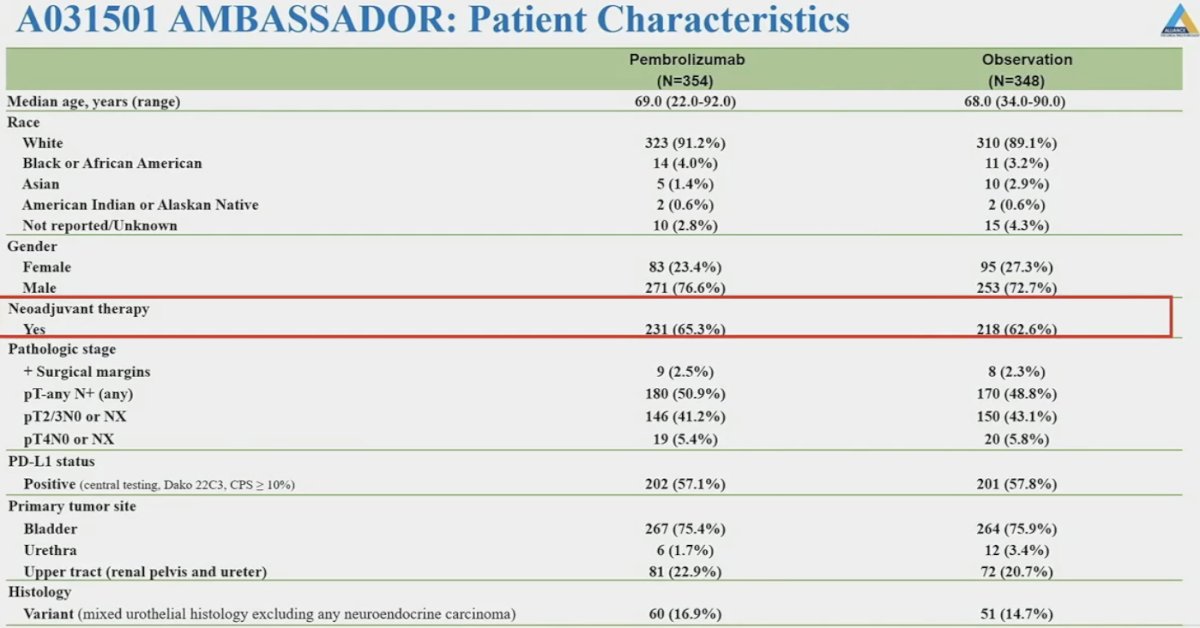
At a median follow-up of 22 months, pembrolizumab improved disease-free survival in the intent-to-treat population from 14 to 29 months (HR: 0.69, 95% CI: 0.54 to 0.87, p=0.001).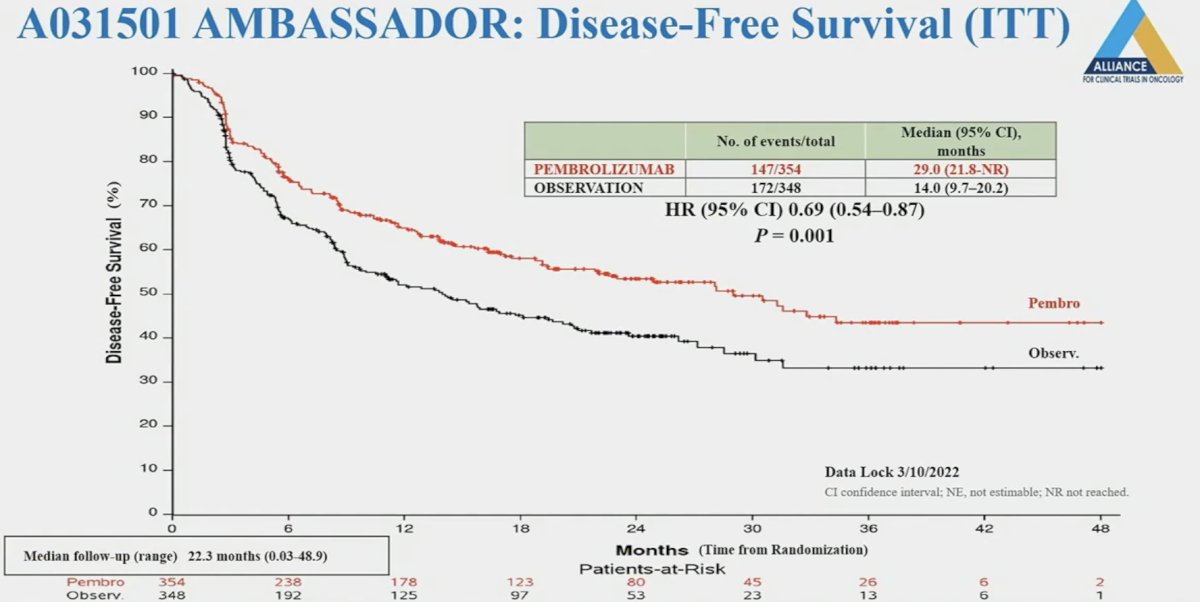
On subgroup analyses, consistent disease-free survival benefits were observed with pembrolizumab, with the notable exception of an upper tract primary tumor site (HR: 1.05 – this is similar to that observed with nivolumab in the adjuvant setting).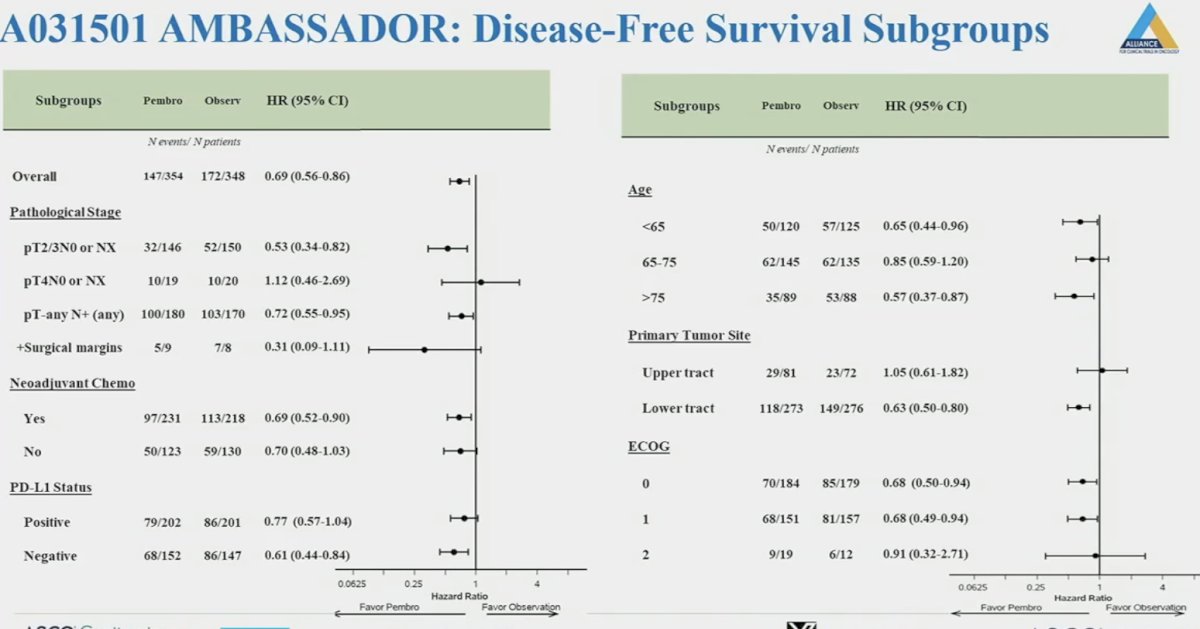
PD-L1 status was prognostic, but not predictive of treatment response, for DFS outcomes, as summarized below: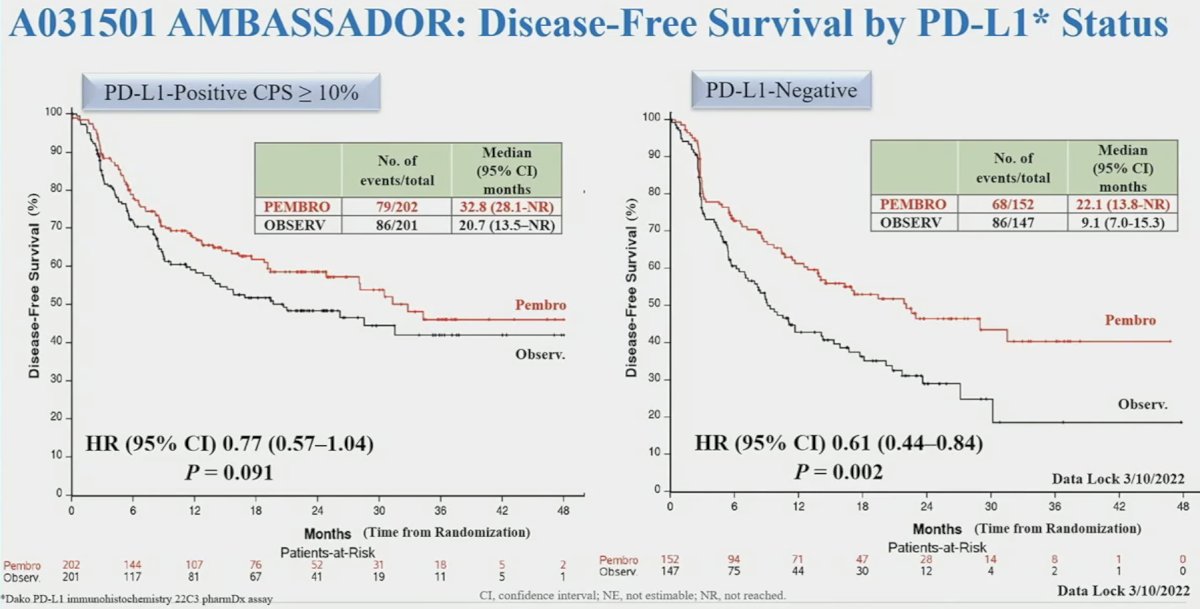
To date, the interim overall survival analyses do not demonstrate a benefit for adjuvant pembrolizumab (HR: 0.98, 95% CI: 0.76 – 1.26, p=0.88).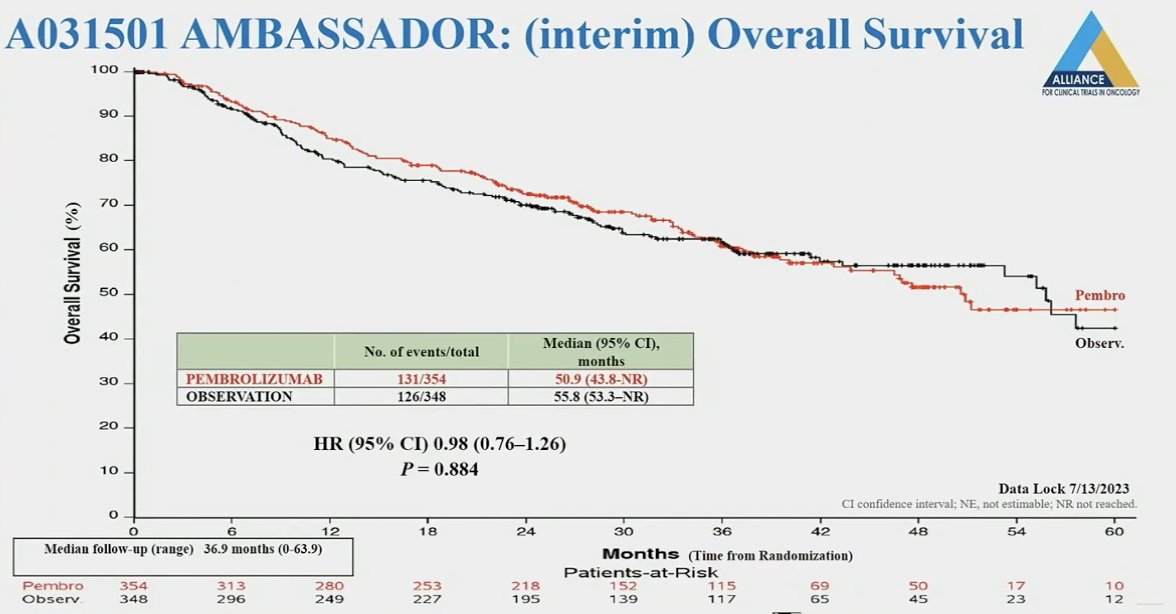
No clear signal for an overall survival benefit by subgroup analysis was observed:
Similarly, PD-L1 was not a predictive biomarker of overall survival response. However, patients who were PD-L1 positive had better median overall survival, highlighting the prognostic nature of this biomarker.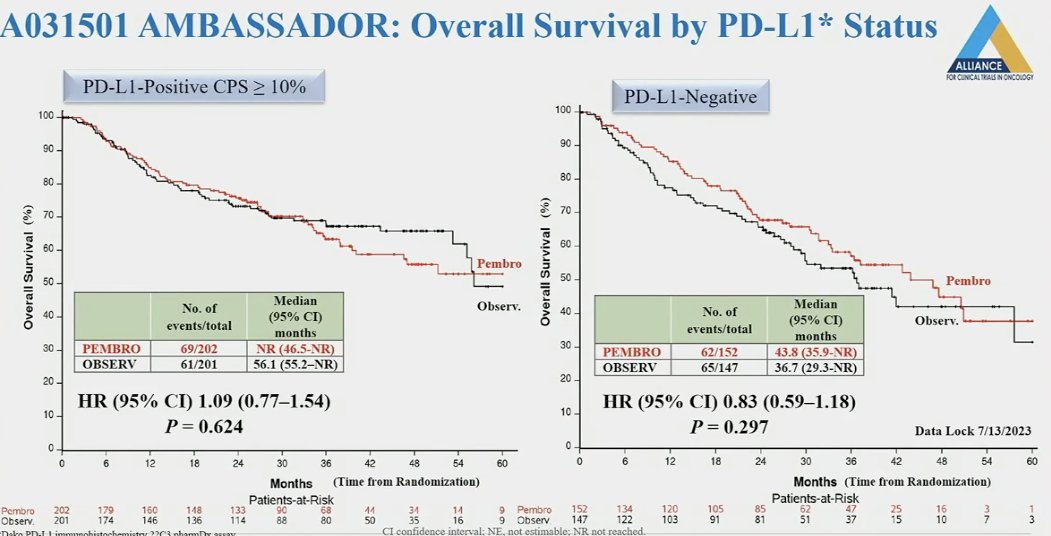
Other systemic therapies were received more frequently in the observation arm patients (35% versus 28%), as summarized below. Notably, 22% of patients post-DFS in the observation arm received checkpoint inhibitors, compared to only 5% in the pembrolizumab arm.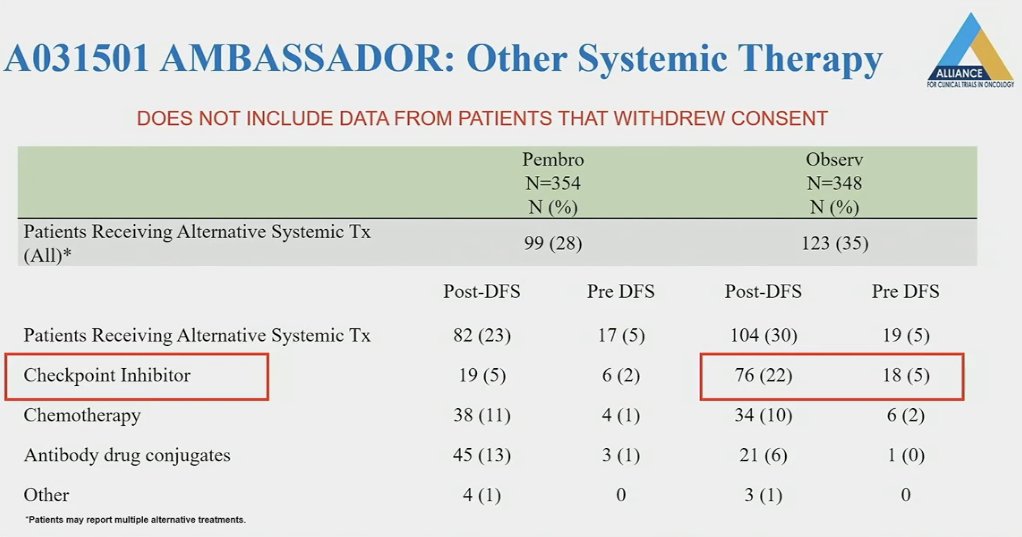
Grade 3 adverse events were observed in 48% and 32% of patients in the pembrolizumab and observation arms, respectively.
The most common treatment-related adverse events were fatigue, pruritis, diarrhea, and hypothyroidism.
Dr. Apolo concluded that:
- Adjuvant pembrolizumab demonstrated a statistically significant and clinically meaningful improvement in disease-free survival compared to observation alone in patients with high-risk muscle invasive urothelial carcinoma after radical surgery, regardless of PD-L1 status. The trial met its efficacy endpoint and is a ‘positive’ trial.
- The overall survival endpoint was not met at the interim analysis. It may have been impacted by the high number of patients on the observation arm receiving a checkpoint inhibitor. Additional events are needed for the final analysis.
- PD-L1 positivity using the combined positive score (CPS) was associated with a better prognosis but was not predictive of treatment efficacy. PD-L1 status should not be used to select patients for treatment.
- Pembrolizumab was tolerable with no new safety signals
- Additional follow-up is ongoing for the final DFS/OS, ctDNA analyses, and additional correlatives.
- These results support adjuvant pembrolizumab as a new therapeutic option for patients with muscle invasive urothelial carcinoma with high risk for recurrence.
Presented by: Andrea B. Apolo, MD, Chief, Bladder Cancer Section Genitourinary Malignancies Branch Center for Cancer Research, National Cancer Institute, Bethesda, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related Content:
Adjuvant Pembrolizumab in Urothelial Carcinoma: AMBASSADOR Study Findings - Andrea Apolo
VISTA as a New Immunotherapy Target


