(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium featured a urothelial carcinoma session and a presentation by Dr. Joseph Jacob discussing reasons for refusal of or ineligibility for radical cystectomy in patients with BCG–unresponsive high-risk non–muscle-invasive bladder cancer (NMIBC) from the SunRISe-1 study. Radical cystectomy is the recommended treatment option for patients with BCG-unresponsive high risk NMIBC. However, radical cystectomy is associated with significant risk of morbidity, mortality, and impact on quality of life, thus some patients may refuse/be ineligible for radical cystectomy. In a systemic review of 160 real-world studies, <20% of patients with high risk NMIBC recurrent after BCG underwent radical cystectomy.1 For radical cystectomy-ineligible patients, bladder-sparing treatment is recommended.
TAR-200, a novel intravesical drug delivery system that provides sustained release of gemcitabine within the bladder, is under investigation in patients with BCG-unresponsive high risk NMIBC who are ineligible for or refuse radical cystectomy in the ongoing phase 2b SunRISe-1 study. Preliminary results, presented by Dr. Daneshmand at AUA 2023, showed a promising complete response rate of 73% and durable responses in patients with BCG-unresponsive high risk NMIBC treated with TAR-200. At GU ASCO 2024, Dr. Jacob reported reasons for refusal or ineligibility for radical cystectomy in patients enrolled in the TAR-200 monotherapy cohort of SunRISe-1.
SunRISe-1 is evaluating the efficacy and safety of TAR-200 + cetrelimab (anti–PD-1) (Cohort 1), TAR-200 alone (Cohort 2), or cetrelimab alone (Cohort 3):
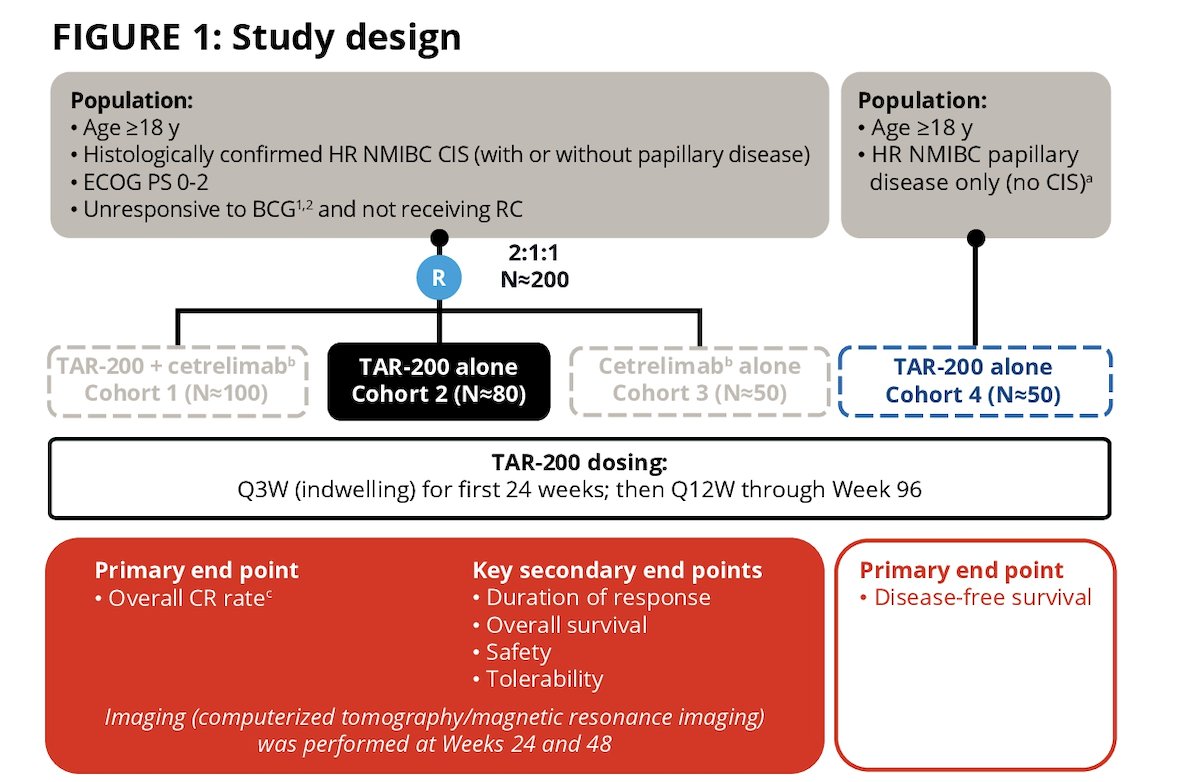
Patients ≥18 years of age with histologically confirmed carcinoma in situ (CIS) ± papillary disease (T1, high-grade Ta) who completed adequate BCG and recurred ≤12 months since last BCG dose with ECOG performance status of 0-2 are randomized to Cohort 1, 2, or 3. As of Amendment 4, patients with papillary disease only will be enrolled in Cohort 4 with TAR-200 alone. The primary endpoint is overall complete response rate at any time. Refusal or ineligibility for radical cystectomy was documented in the electronic case report form.
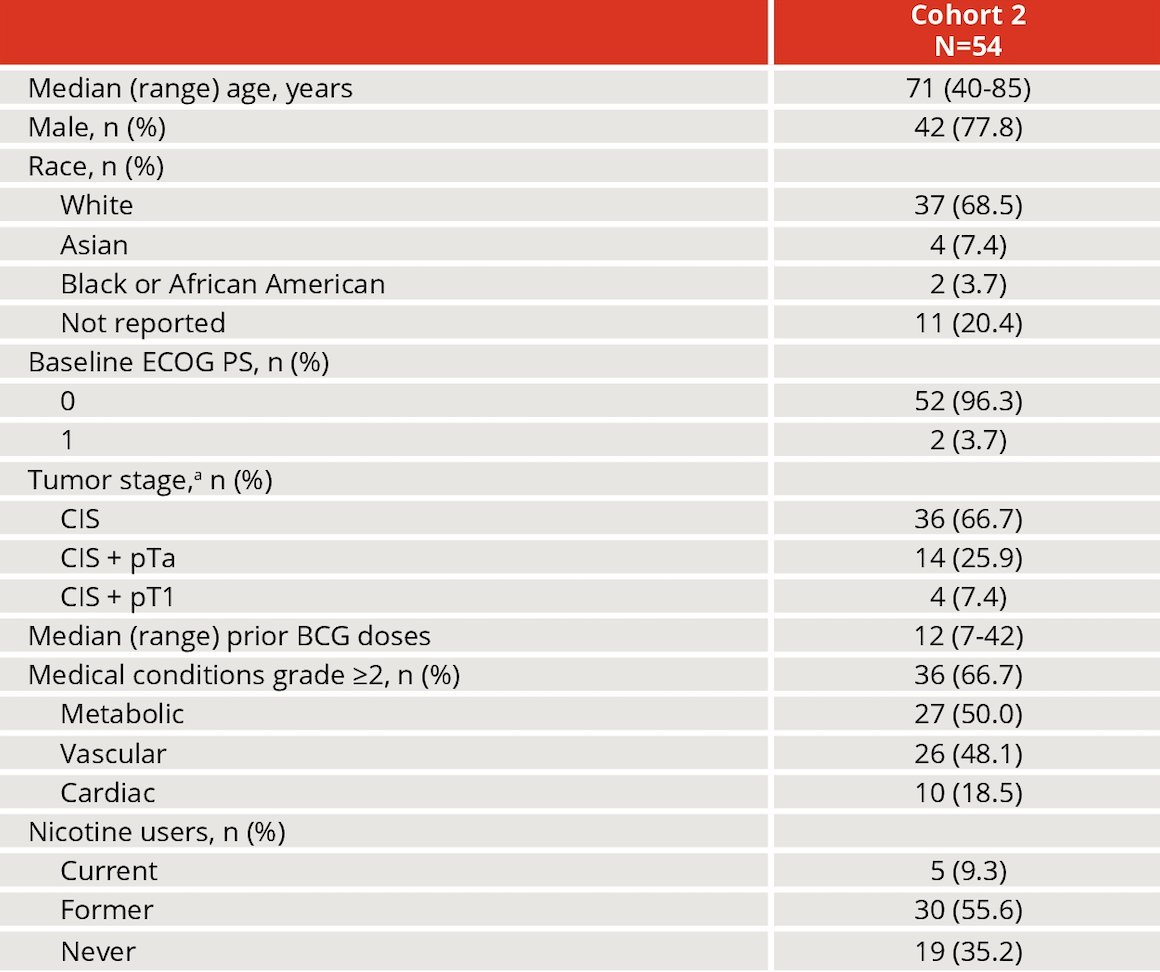
As of August 24, 2023, 54 patients were treated in Cohort 2. The median age was 71 years (range 40-85), and patients had a median of 12 (range 7-42) prior BCG doses with a median of 3 months (range 0-22.0) from last BCG dose to recurrence. Most patients (96%) had ECOG performance status 0, and 33% had CIS with papillary disease. There 50% of patients that had a median history of grade ≥2 metabolic conditions, 48% with vascular, and 19% of patients with a history of cardiac conditions, and 9% were current nicotine users:
Overall, 51 (94%) patients refused radical cystectomy, of which 3 (6%) patients were ineligible:
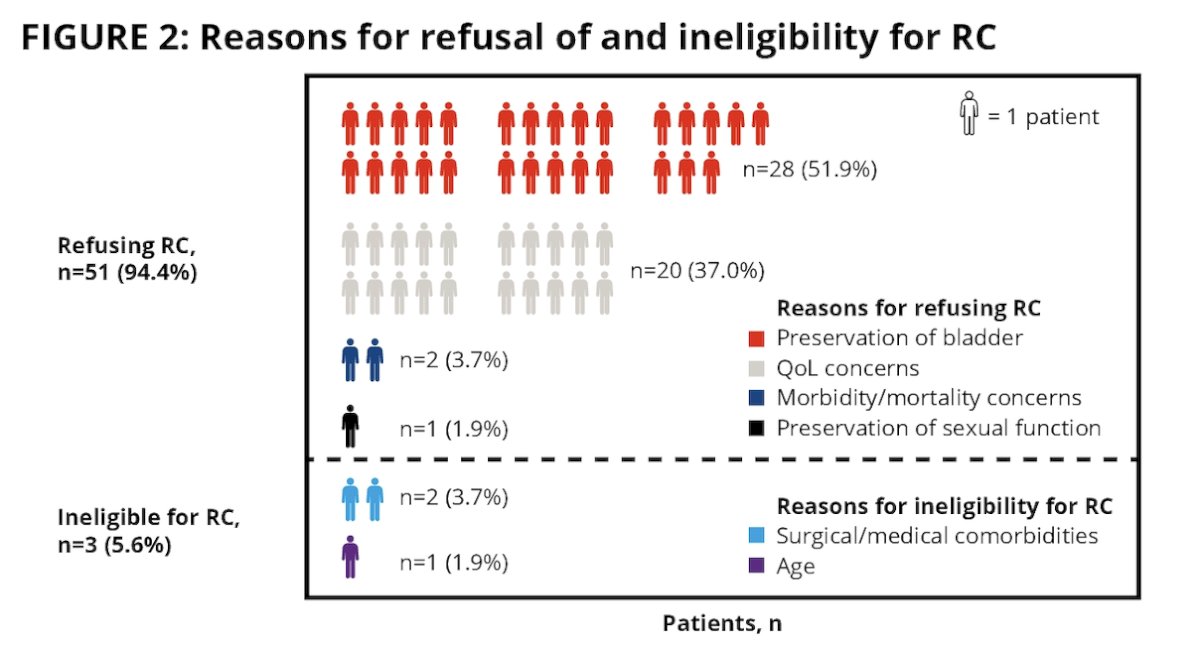
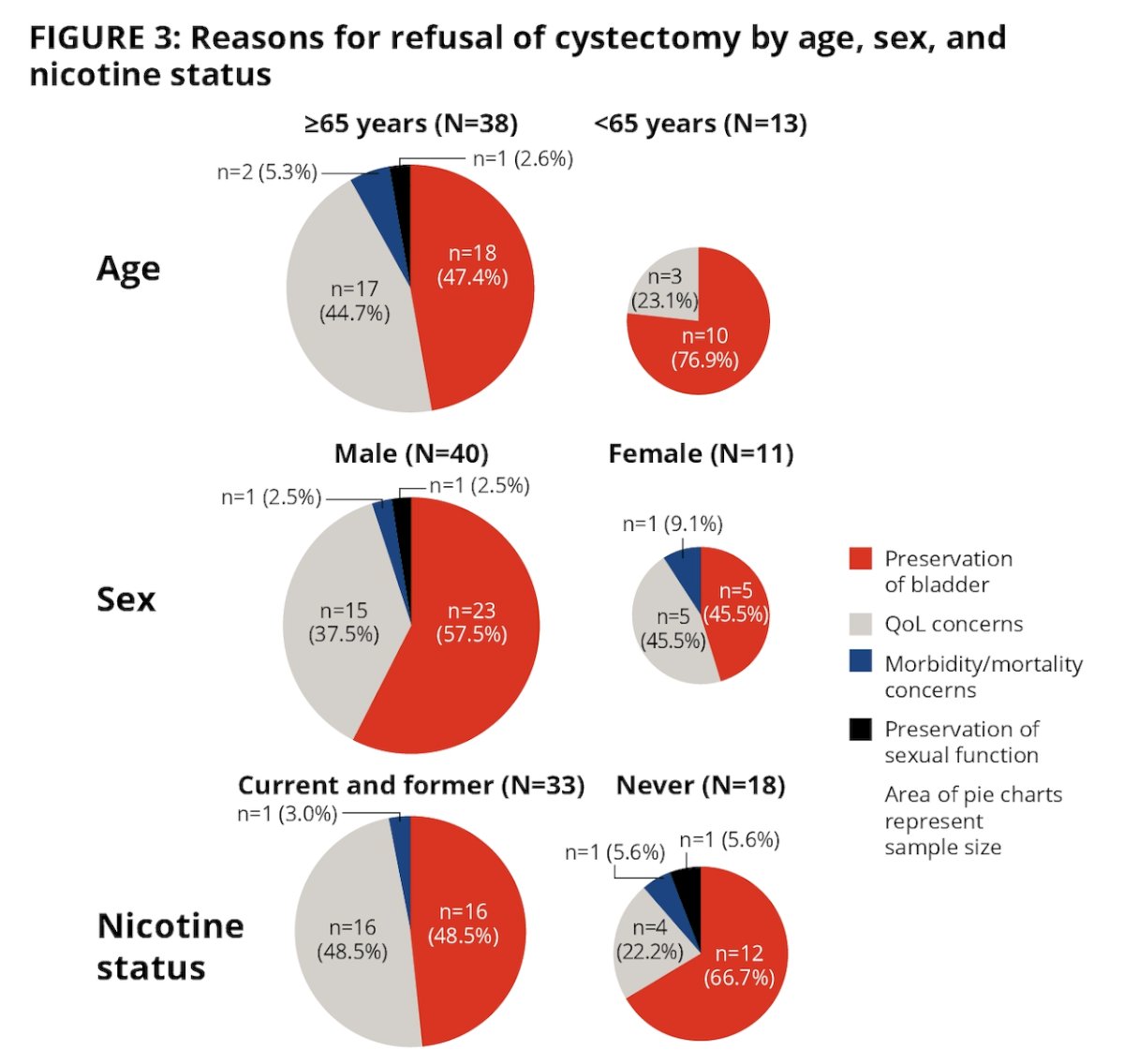
The most common reason for refusal was a preference for bladder preservation (52%), followed by concern about quality of life (37%):
Patients were ineligible for radical cystectomy due to medical/surgical comorbidities in 4% of case and secondary to age in 2% of cases. No responders underwent radical cystectomy at the time of data cutoff (n = 23).
Dr. Jacob concluded his presentation by discussing reasons for refusal of or ineligibility for radical cystectomy in patients with BCG–unresponsive high-risk NMIBC from the SunRISe-1 study with the following take-home points:
- Efficacy and safety data from SunRISe-1 support the ongoing investigation of TAR-200 in patients with BCG-unresponsive high-risk NMIBC
- Over 90% of the patients with high risk NMIBC CIS recurrent after BCG enrolled in Cohort 2 of SunRISe-1 refused radical cystectomy
- The most common reasons for refusal of radical cystectomy were bladder preservation and quality of life concerns
- A small number of patients in the trial were ineligible for radical cystectomy (age and comorbidities)
Presented by: Joseph M. Jacob, MD, Department of Urology, Upstate Medical University, Syracuse, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


