(UroToday.com) The 2024 GU ASCO annual meeting featured a urothelial carcinoma session and a presentation by Dr. Shilpa Gupta discussing the application of artificial intelligence features of nuclear morphology from BLASST-1 of nivolumab, gemcitabine, and cisplatin in patients with MIBC undergoing cystectomy. BLASST-1 is a multi-center phase II trial evaluating neoadjuvant nivolumab with gemcitabine-cisplatin for patients with MIBC undergoing radical cystectomy. In BLASST-1, 41 patients with MIBC (cT2-T4a, N≤1, M0) were enrolled between February 2018 and June 2019: cT2N0 90%, cT3N0 7%, cT4N1 3%. Patients received cisplatin (70mg/m2) IV on day 1, gemcitabine (1000mg/m2) on day 1, day 8, and nivolumab (360 mg) IV on day 8 every 21 days for 4 cycles followed by radical cystectomy within 8 weeks. The primary endpoint was pathologic downstaging (≤pT1N0). Safety, relapse-free survival, progression-free survival, and biomarker analyses were secondary endpoints. The pathologic downstaging rate was 65.8%, the pathologic complete response (≤pT is N0) rate was 49%, and there were no safety concerns or delays to radical cystectomy.
Morphometric characteristics of the cell nucleus can be used to assess bladder cancer grading and gain insights into cellular functionalities. In this study, Dr. Gupta and colleagues sought to evaluate the ability of an artificial intelligence model to identify non-responders to neoadjuvant chemo-immunotherapy in the BLASST-1 cohort based on computerized image features of nuclear morphology and architecture on pre-treatment TURBT tissues.
Of the 41 patients, there was H&E images available for 34 patients, of which 23 had pathologic downstaging and 11 did not have pathologic downstaging; these were classified as responder and non-responder groups. A machine learning model (U-net) was developed and invoked for tumor segmentation on the H&E images from the BLASST-1 cohort. A second machine learning model (HoVer-Net) was employed to segment and classify individual nuclei. A total of 408 features relating to the textural and spatial arrangement of individual cancer nuclei were extracted. The proposed pipeline for artificial intelligence model is highlighted as follows:
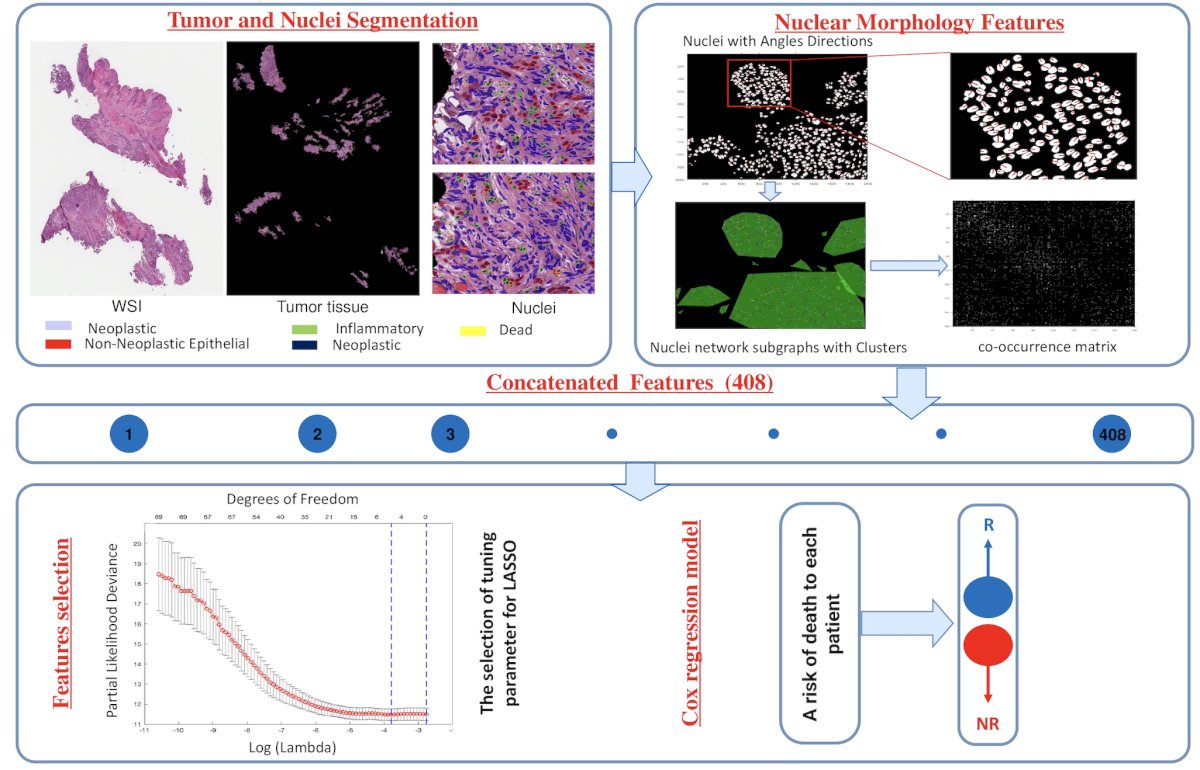
The 17 most significant features, identified through the least absolute shrinkage and selection operator, were used to train a Cox regression model to predict the risk of death using 363 MIBC patients from The Cancer Genome Atlas (TCGA). This Cox model was then applied to assign a risk score to patients in BLASST-1. Using a threshold learned from TCGA patients, the individual patients in BLASST-1 were assigned as either low-risk or high-risk.
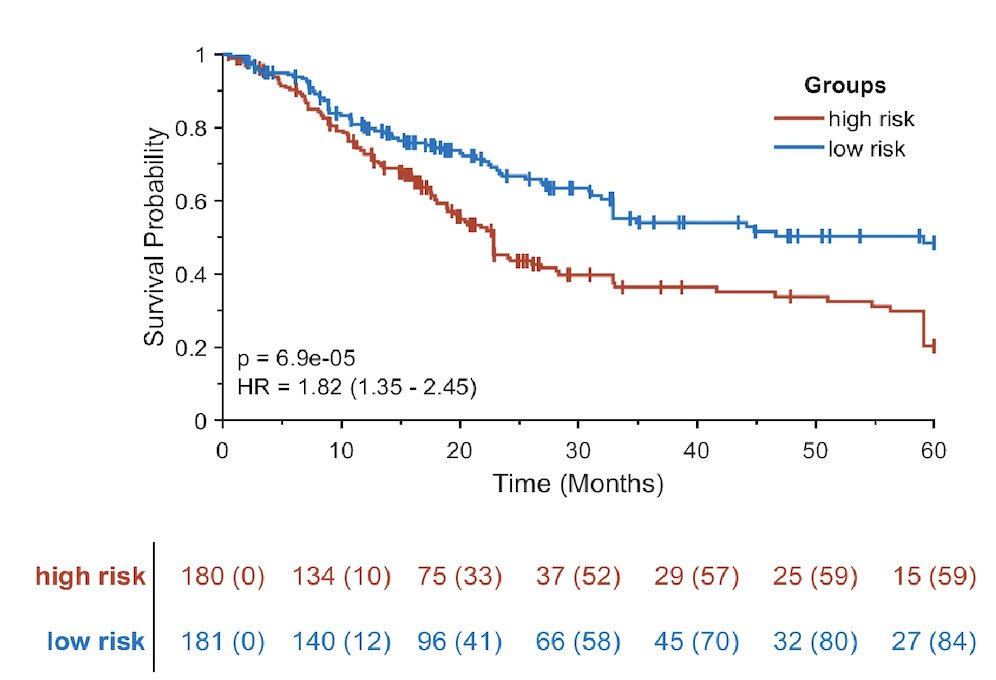
For training the TCGA, the model based on nuclear morphology features was prognostic of overall survival with a hazard ratio of 1.82 (95% DCI 1.35-2.45) as shown:
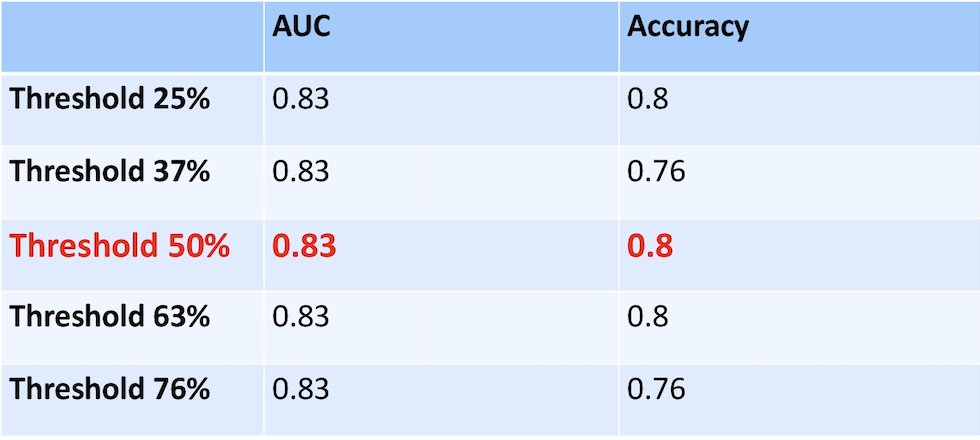
The top identified prognostic features in BLASST-1 described the textural appearance of individual nuclei with more texture. This model accurately predicted pathologic downstaging, with an AUC of 0.83. The following table shows the effect of using thresholds learned from the TCGA patients on AUC and accuracy of the BLASST-1 cohort:
Dr. Gupta concluded her presentation by discussing the application of artificial intelligence features of nuclear morphology from BLASST-1 of nivolumab, gemcitabine, and cisplatin in patients with MIBC undergoing cystectomy with the following take-home points:
- A computerized artificial intelligence model relying on nuclear morphologic and architecture features demonstrated prognostic capability in MIBC within the TCGA dataset and predictive capability for the pathologic downstaging in the BLASST-1 trial
- These findings support further validation studies
- The patients with the same nuclear angle direction within tumor tissue had a high chance of response to the neoadjuvant chemo-immunotherapy combination in the BLASST trial
Presented by: Shilpa Gupta, MD, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


