(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the role of immunotherapy in advanced urothelial carcinoma and a presentation by Dr. William Kim discussing the biologic basis of pairing and sequencing checkpoints with other therapies. Dr. Kim started by asking the question “What do we know about the bladder cancer tumor microenvironment?” Generally, urothelial carcinoma has a high tumor mutational burden, and antigen driven immune responses likely account for immune checkpoint inhibitor responses:
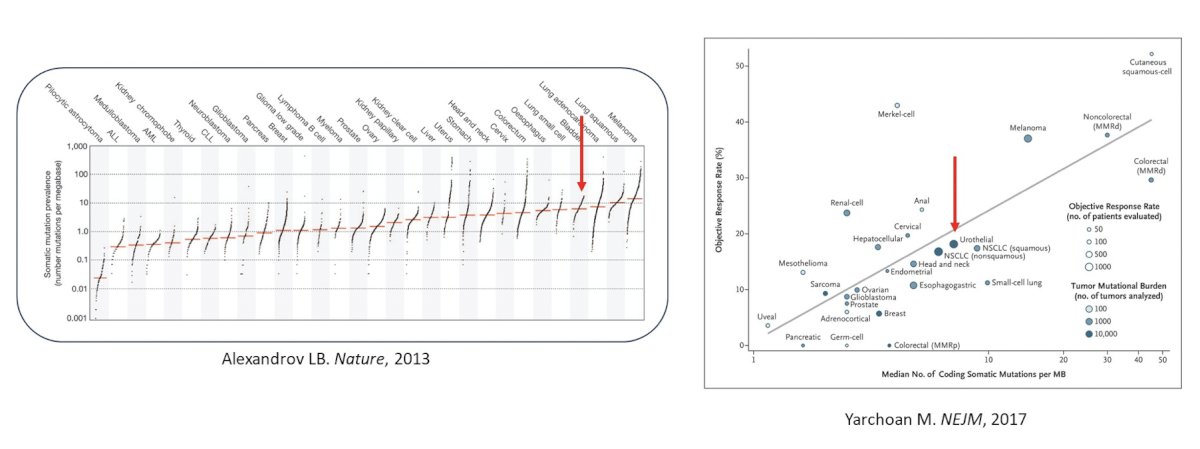
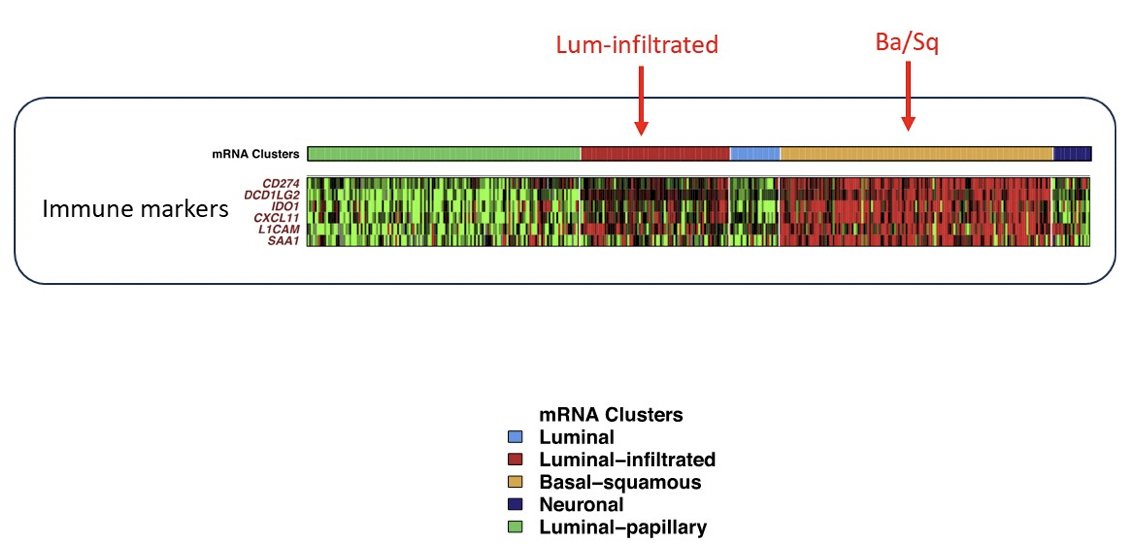
Looking closer at the tumor microenvironment, in the last several years we have developed molecular signatures, for example, luminal-infiltrative and basal/squamous from the TCGA dataset:
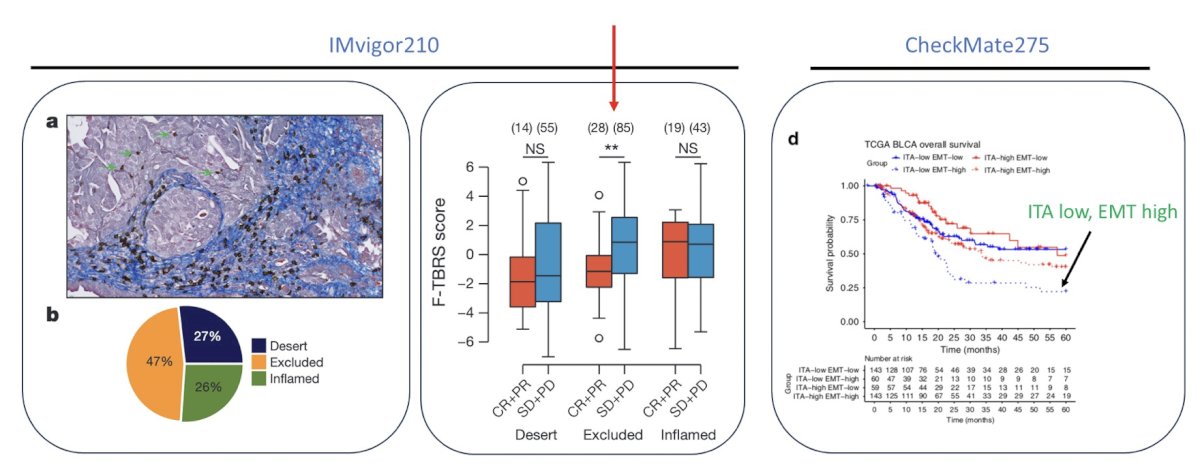
Additionally, we have seen the immunohistochemistry subtypes, including immune desert, excluded, and inflamed tumors, which contextualize the importance of the stroma:
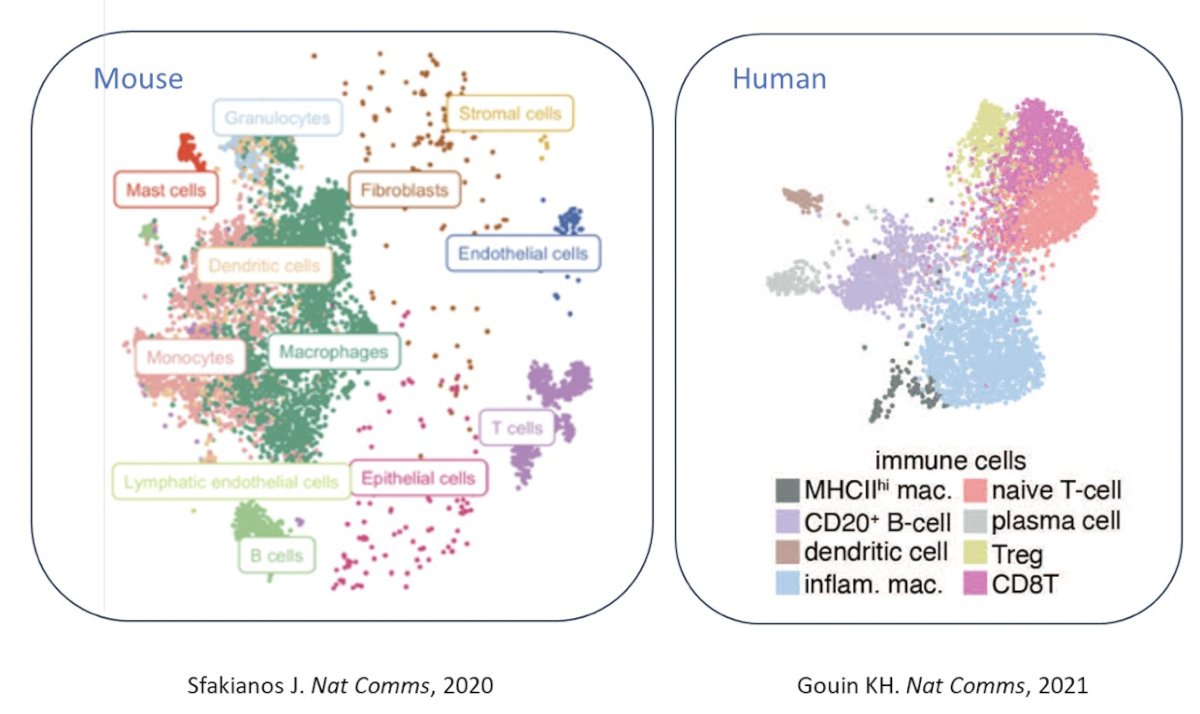
Even more recently, a more refined view of the muscle invasive bladder cancer tumor microenvironment has developed through scRNAseq or snRNAseq. This has shown us that mice and human bladder tumors have reasonably similar immune contexture and that macrophages and T cells appear predominant:
With regards to the intratumoral tumor microenvironment, we are also able to assess heterogeneity through digital special profiling:
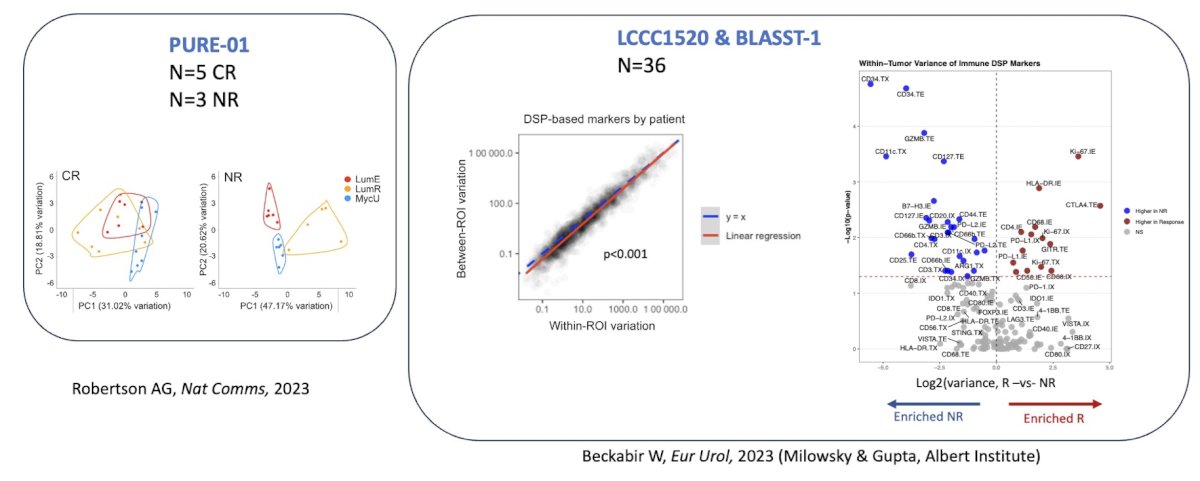
To summarize this section of his talk on the tumor microenvironment, Dr. Kim highlighted that:
- Muscle invasive bladder cancer has a high tumor mutational burden
- There is variability in the T cell inflamed phenotype
- Macrophages and T cells are the predominant cell types that make up the muscle invasive bladder tumor microenvironment
- There is intratumoral heterogeneity with checkpoint and immune cell types that may correspond to immune checkpoint inhibitor response
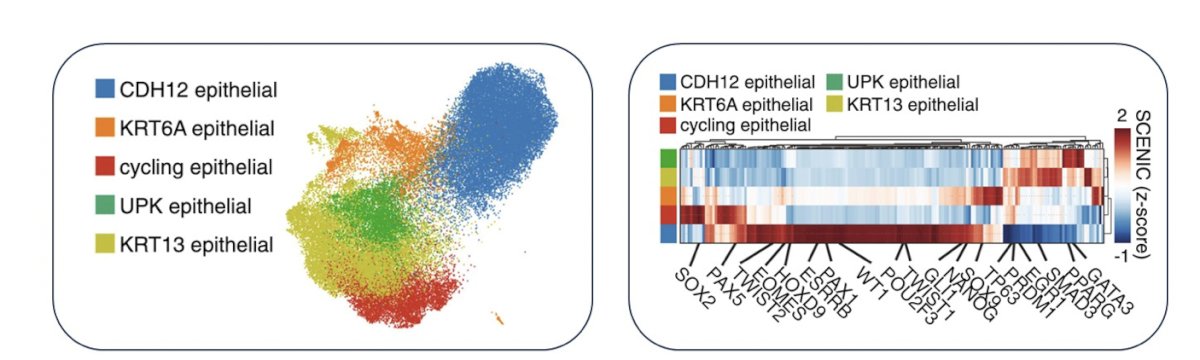
To set the stage for the immune checkpoint inhibitor combinations in muscle invasive bladder cancer, there are several combinations, including (i) chemotherapy + immune checkpoint inhibitors, (ii) antibody drug conjugates + immune checkpoint inhibitors, (iii) FGFR3 inhibition + immune checkpoint inhibitors, (iv) MAPK inhibition + immune checkpoint inhibitors, and (v) NKG2a blockade + immune checkpoint inhibitors. In early work among 30 muscle invasive bladder cancer assessing pre and post neoadjuvant chemotherapy specimens, it was noted that chemotherapy does not have a significant impact on the tumor mutational burden, although subclonal mutations are unique. Additionally, Dr. Kim notes that CDKH12 high cells may represent a chemotherapy resistant population of patients:
Furthermore, luminal tumors have been shown to increase immune gene signatures in response to MVAC, with the following heat map representing changes in IGS (red: higher in post; blue: lower in post):
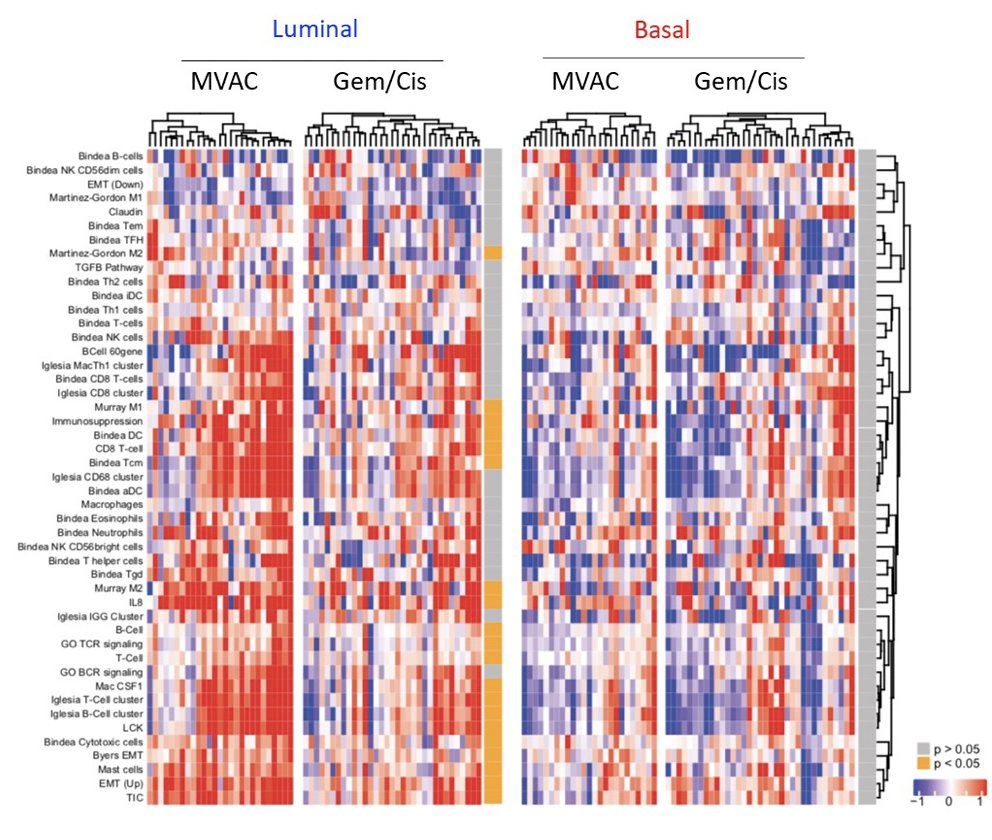
However, to date, we do not know the effectiveness of the immune infiltrate. For gemcitabine + cisplatin chemotherapy for the treatment of luminal tumors, this has been shown to increase expression of stroma-related signatures correlating with resistance to immunotherapy:
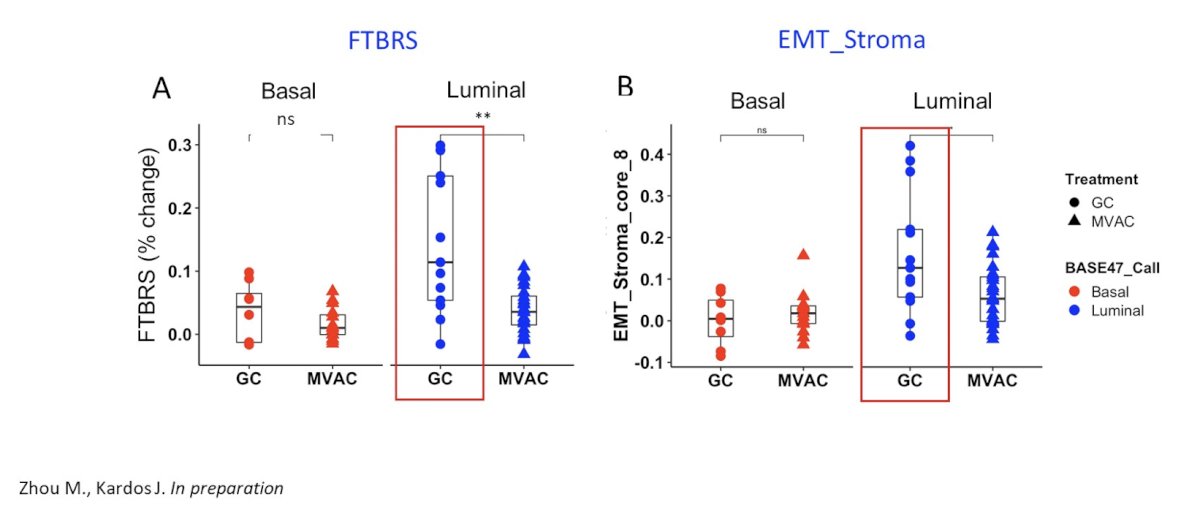
In recently published work from Galsky and colleagues,1 they investigated the immunomodulatory effects of cisplatin as a potential explanation for the more favorable effects of atezolizumab combined with gemcitabine + cisplatin versus gemcitabine + carboplatin in IMvigor130. In this analysis, they found that improved outcomes with gemcitabine + cisplatin versus gemcitabine + carboplatin are primarily observed in patients with pretreatment tumors exhibiting features of restrained adaptive immunity. In addition, gemcitabine + cisplatin versus gemcitabine + carboplatin ± atezolizumab induces transcriptional changes in circulating immune cells, including upregulation of antigen presentation and T cell activation programs:
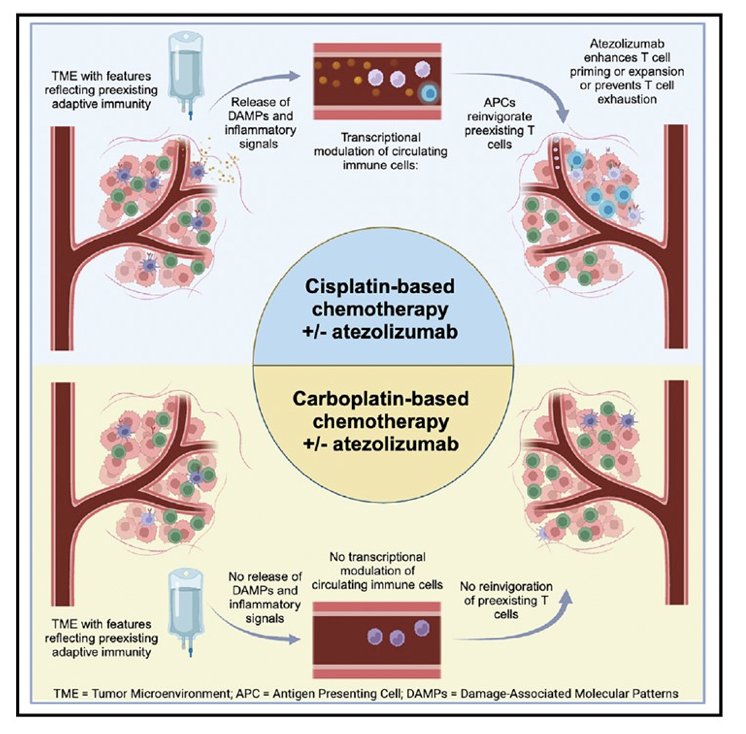
Thus, Dr. Kim emphasized that not all chemotherapy is created equal and we need to be thoughtful about our pairings.
Presented at ESMO 2023, EV-302/KEYNOTE-A39 assessing enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated locally advanced and metastatic urothelial carcinoma completely changed the paradigm of first line treatment of metastatic urothelial carcinoma. Overall survival was nearly double in the enfortumab vedotin + pembrolizumab arm, with median survivals of 31.5 and 16.1 months in the enfortumab vedotin + pembrolizumab and chemotherapy arms, respectively (HR 0.47, 95% CI 0.38 – 0.58, p < 0.00001):
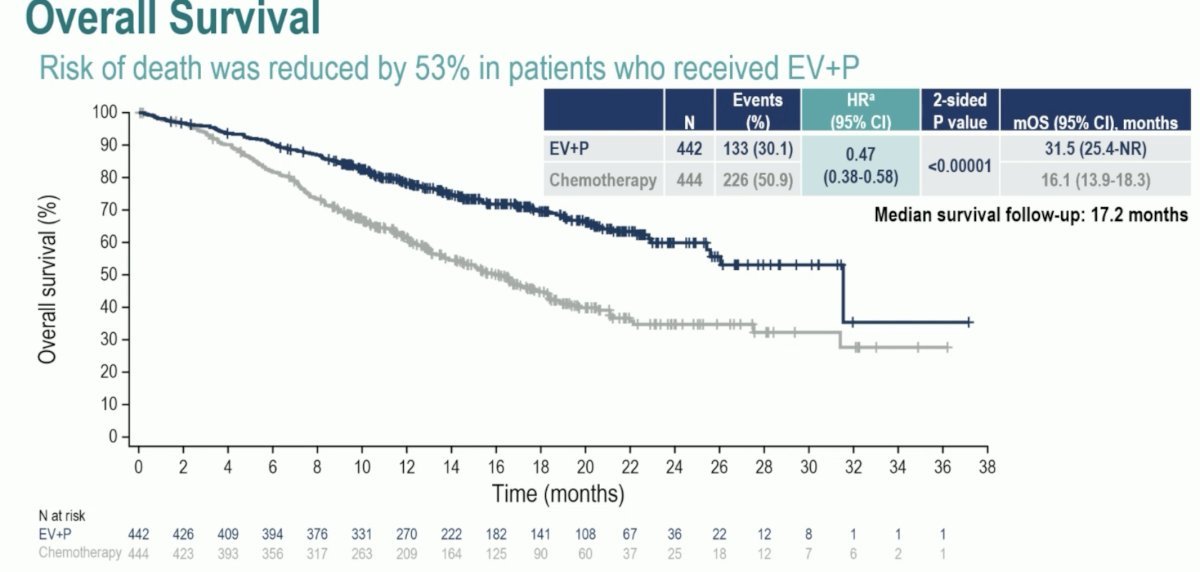
So, why does enfortumab vedotin + pembrolizumab work so well?
- Is it because of immunogenic cell death?
- Does enfortumab vedotin and pembrolizumab target different populations?
Dr. Kim notes that NECTIN4 is expressed most highly in luminal-like cells and that pembrolizumab may work well on basal-like cells, although this is somewhat controversial.
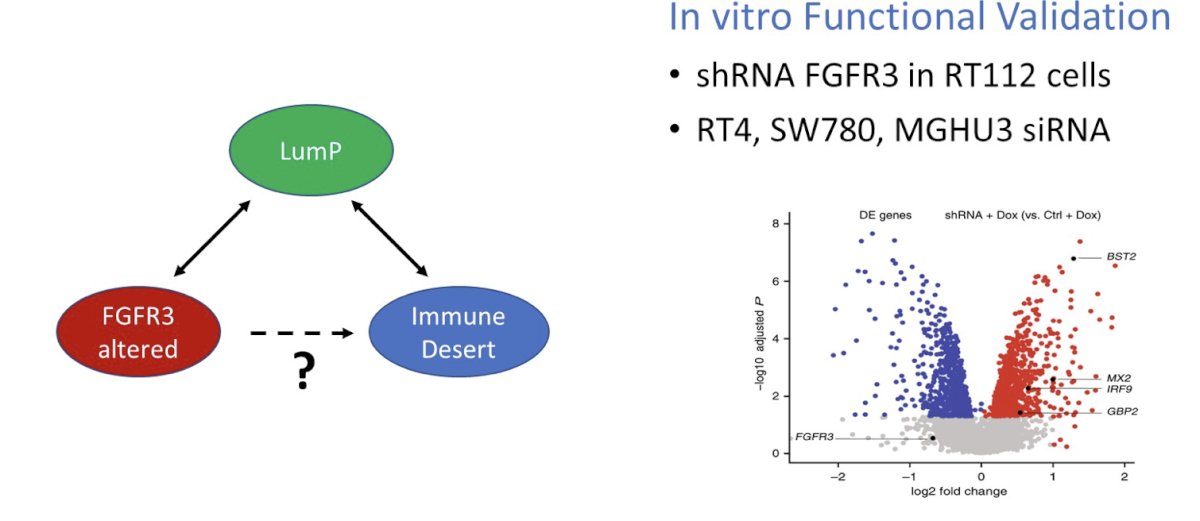
With regards to FGFR inhibition and immune checkpoint inhibitors, the phase 2 Norse trial tested erdafitinib versus erdafitinib + cetrilumab, noting a median progression free survival of 10.97 months (95% CI 5.45 – 13.63) for erdafitinib + cetrilumab versus 5.62 months (95% CI 4.34 – 7.36) for erdafitinib alone. But, to what extent is FGFR3 activation responsible for the immune desert phenotype we see in luminal-papillary tumors?
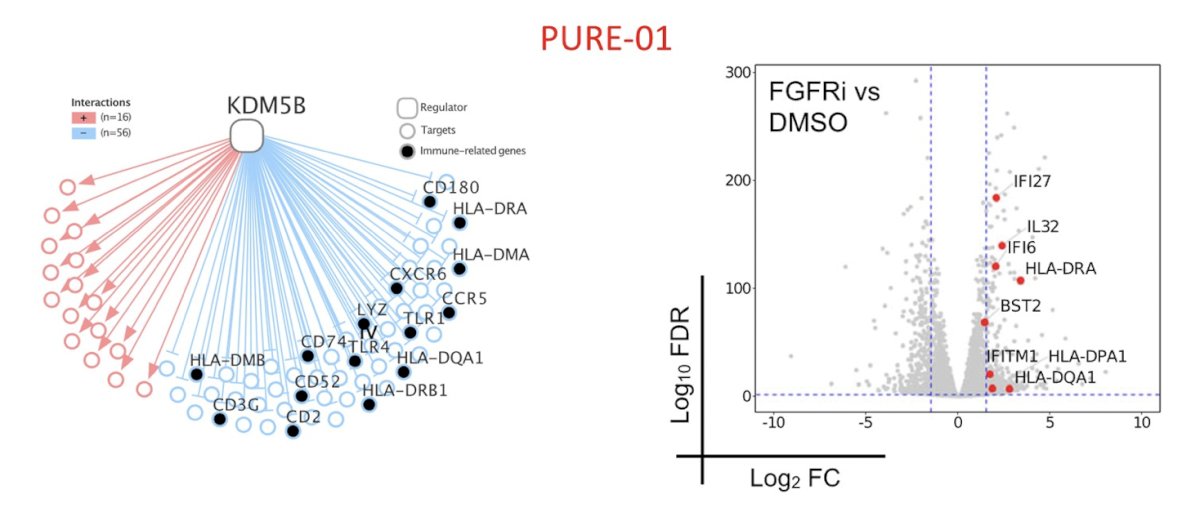
In the PURE-01 trial, analyses found that regulon assessment of non-responding tumors identified KDM5B and FGFR3. Thus, FGFR3 activation suppressed interferon signaling in the cell in an autonomous manner:
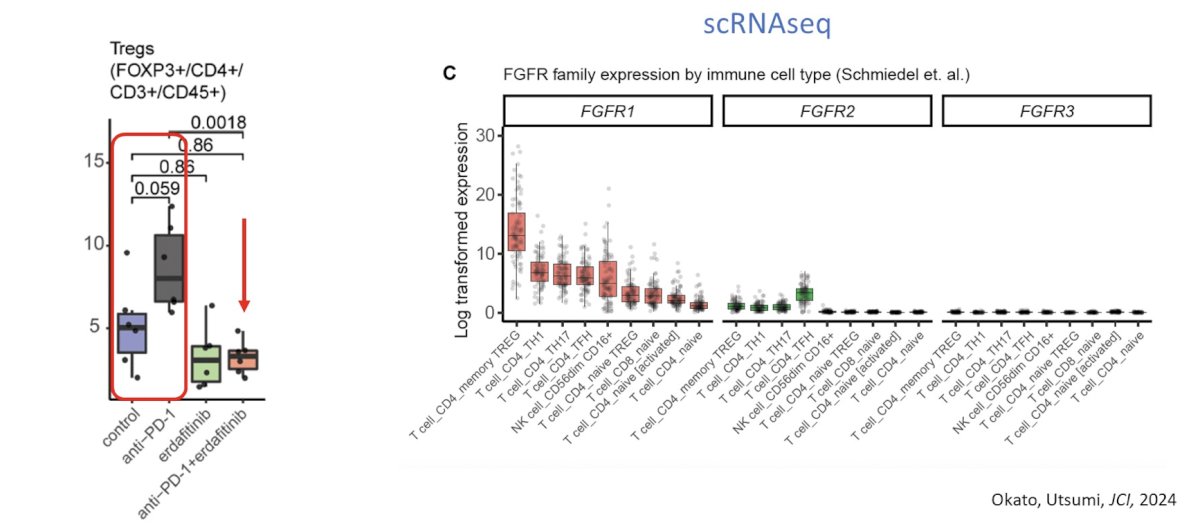
Dr. Kim also noted that anti-PD-1 promotes Treg expansion, which can be abrogated by co-treatment with erdafitinib based on the following since cell RNAseq analysis:
Dr. Kim then discussed the MAPK inhibition + immune checkpoint inhibitor combination. Several studies have shown that MAPK inhibition can cooperative with immune checkpoint inhibitors, as highlighted below:
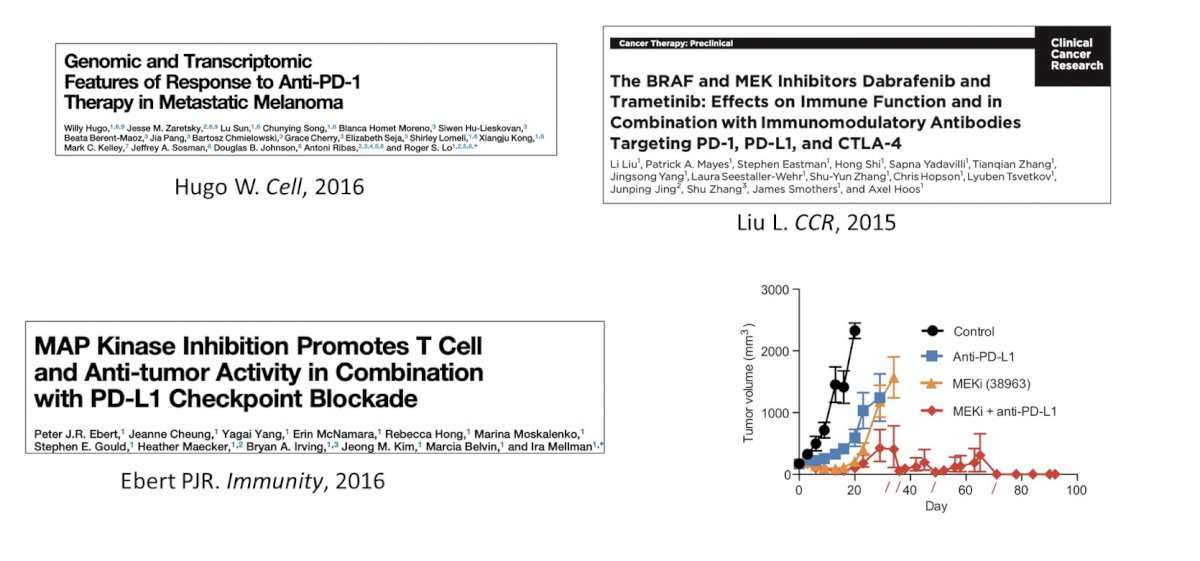
Additionally, small phase 2 trials have shown objective response rates ranging from as low as 7% to upwards of 25%. With regards to NKG2A + immune checkpoint inhibitors, HLA-E is a non-classical class 1 MHC molecular that binds NKG2A on NK cells and CD8+ T cells. Subsequently, NKG2A blockade reinvigorates CD8+, and NKG2A+ T cells, which can kill HLA-E expressing tumors cells. Dr. Kim notes that many NKG2A+ cells are PD1+ and thus investigation of combination therapy is warranted.
Finally, NR tumors are known to be enriched in (i) the consensus stroma-rich subtype, (ii) M2 macrophage signature, (iii) B cell signature, and (iv) DC signature. Thus, we may be able to use these characteristics to help identify potentially modifiable features.
Dr. Kim concluded his presentation by discussing the biologic basis of pairing and sequencing checkpoints with other therapy with the following take-home points:
- The tumor microenvironment of bladder cancer metastases is heterogeneous
- Prior therapy can remodel the tumor microenvironment of bladder
- There is much to learn from spatial biology
- The tumor microenvironment of muscle invasive bladder cancer is heterogeneous and loosely correlated with RNA expression subtypes
- There is rationale for immune checkpoint inhibitor combination therapy, but most combinations have yet to be proven
- Lessons from the NR suggest immune desert, Consensus stroma-rich subtype, and proliferative tumors may circumvent immune checkpoint inhibitor efficacy
Presented by: William Y. Kim, MD, The University of North Carolina at Chapel Hill, Chapel Hill, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Galsky MD, Guan X, Rishipathak D, et al. Immunomodulatory effects and improved outcomes with cisplatin- versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer. Cell Rep Med. 2024 Jan 17:101393.


