(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the role of immunotherapy in advanced urothelial carcinoma and a presentation by Dr. Monty Pal discussing what we can learn from other disease sites regarding the implications of checkpoint therapy in urothelial carcinoma. Dr. Pal emphasized that the best thing to do when handling tough topics is to use the “phone a friend” approach, specifically when there are so many medical oncology experts across the globe:

The first take-away point from other disciplines is that adjuvant therapy is good, but neoadjuvant therapy may possibly be better. In the field of melanoma, Dr. Pal notes that Dr. Sapna Patel (University of Texas MD Anderson Cancer Center) states “It’s not just what you give, it’s when you give it. The S1801 study demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.” The trial design for S1801 is as follows:
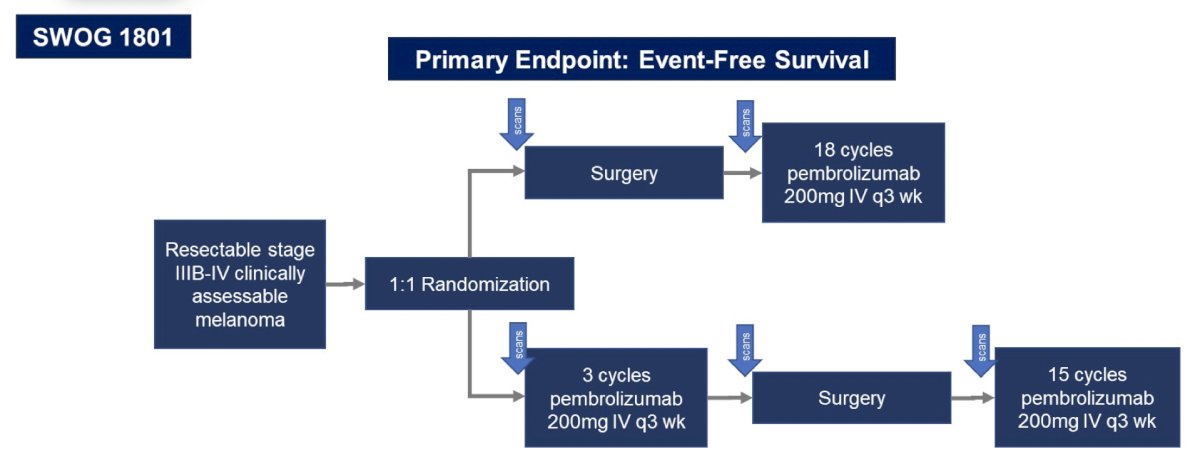
Based on event-free survival, there is a clear benefit for the neoadjuvant-adjuvant group versus the adjuvant-only group in melanoma:

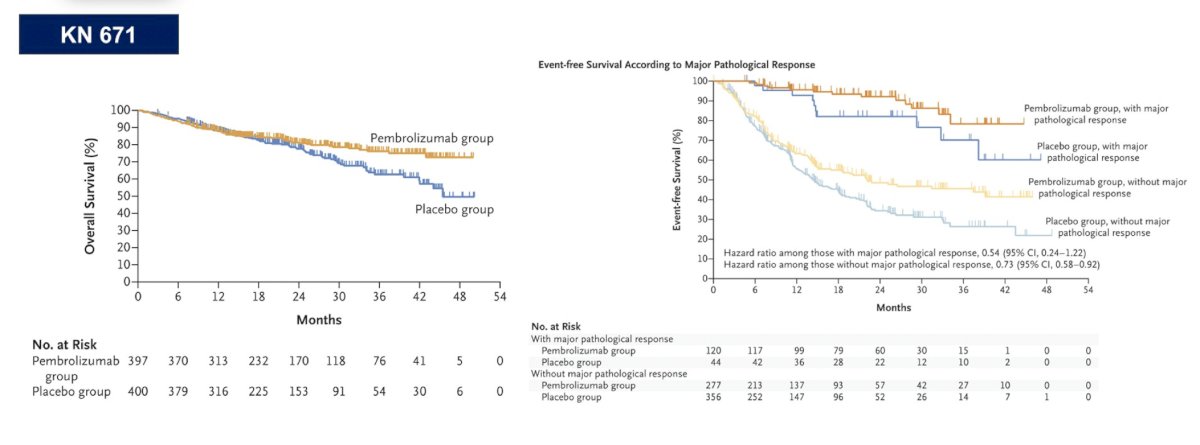
In the field of lung cancer, Dr. Pal highlighted Dr. Heather Wakelee (Stanford University School of Medicine) who states “In lung cancer, the only definitive overall survival data we have is KEYNOTE 671 with both neoadjuvant with chemotherapy and adjuvant pembrolizumab.” The trial design for KEYNOTE 671 is as follows:

Based on the overall survival curves and those receiving pembrolizumab with major pathologic response in the event-free survival curves, there is a clear benefit with pembrolizumab + chemotherapy in the neoadjuvant setting followed by adjuvant pembrolizumab:
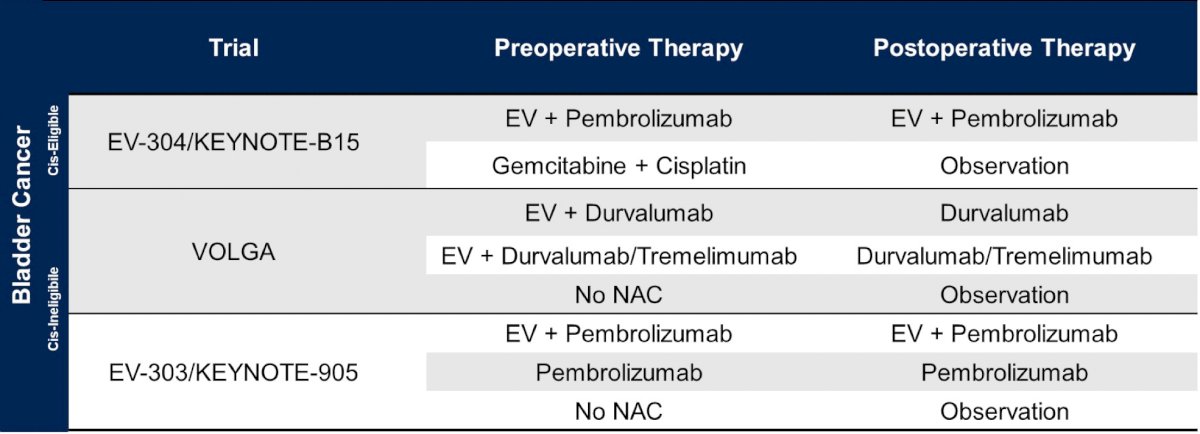
In bladder cancer, Dr. Pal highlighted that there are several important trials assessing pre- and post-operative therapy, including EV-304/KEYNOTE-B15, VOLGA, and EV303/KEYNOTE-905:
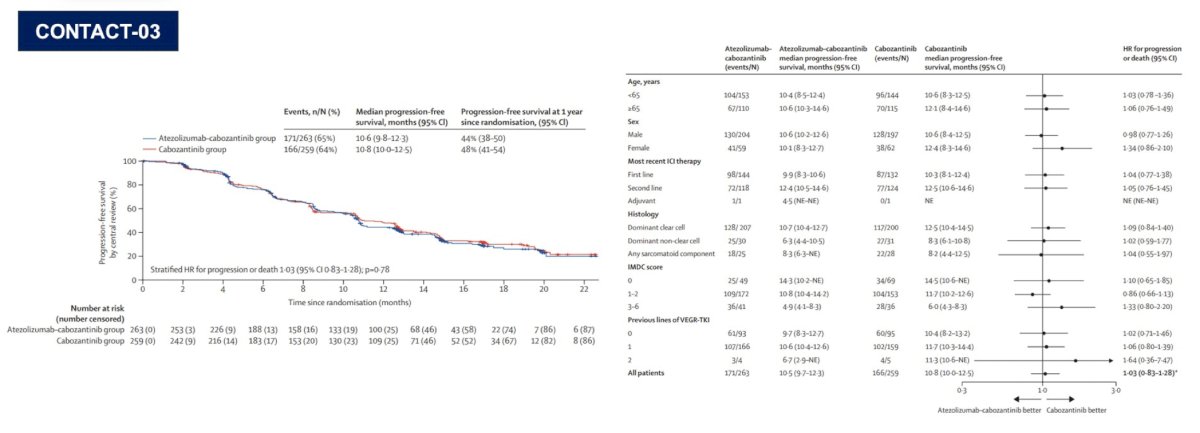
The second take-away point from other disciplines is that optimal sequencing is of paramount importance. In kidney cancer, Dr. Pal highlights Dr. Toni Choueiri (Dana-Farber Cancer Institute) who states “CONTACT-03 highlights the importance of randomized, prospective assessment of rechallenge with checkpoint inhibitors in renal cell carcinoma and potentially other tumor types.” The CONTACT-03 trial design,1 among patients that have progressed on previous immune checkpoint inhibitor treatment, is as follows:
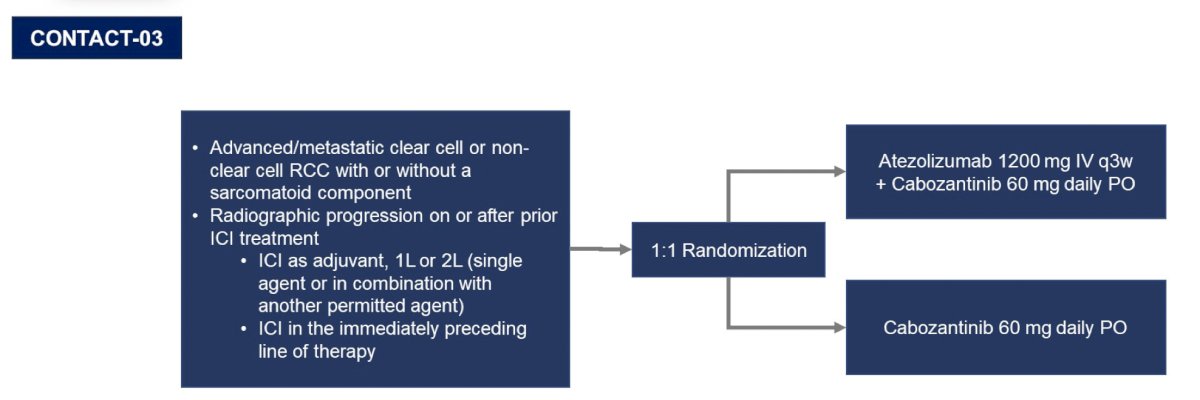
As we know, the CONTACT-03 trial was negative, but perhaps taught us lessons with regards to sequencing in the immunotherapy era, and ultimately making sure to conduct these sequencing trials:
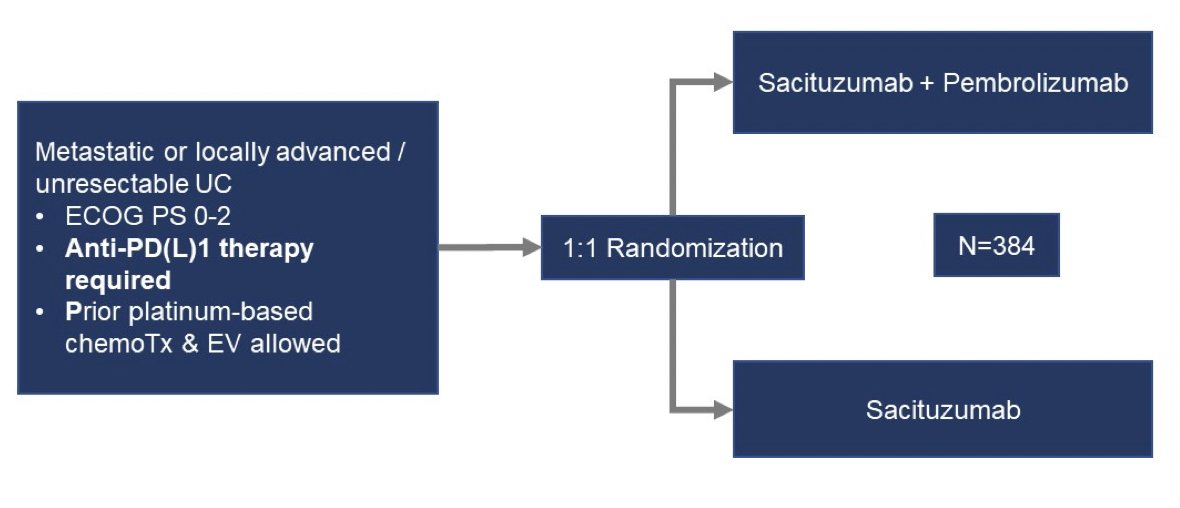
Regarding bladder cancer, Dr. Pal highlighted that there are several trials post-treatment assessing combination therapies, including sacituzumab + pembrolizumab after prior platinum exposure in the TROPHY-U-01 trial, and ECOG-ACRIN 8231 assessing sacituzumab + pembrolizumab versus sacituzumab among metastatic urothelial carcinoma patients that previously received anti-PD(L)1 therapy:
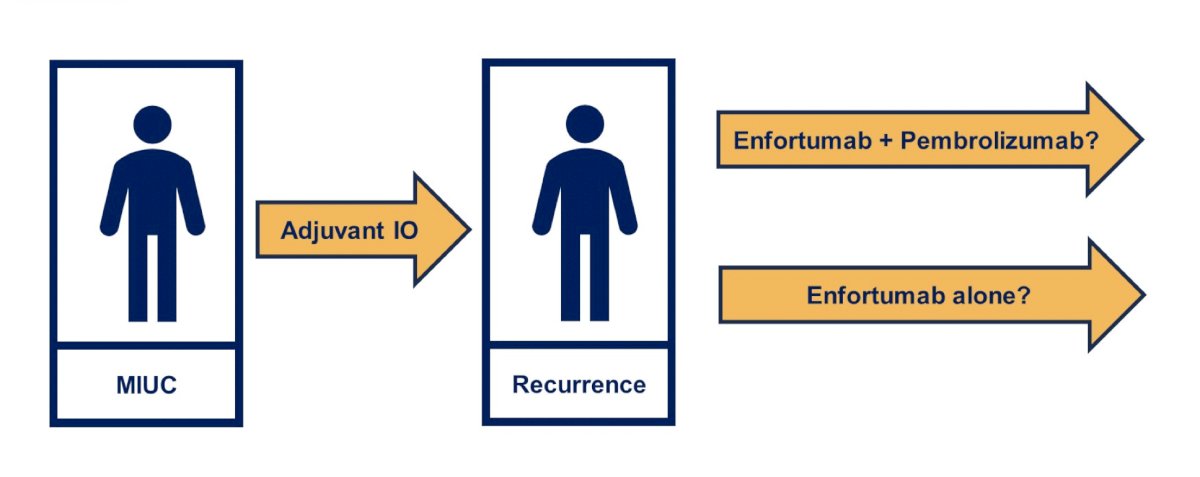
Perhaps the most important trial, given the results of EV-302 trial presented at ESMO 2023 showing substantial benefit of enfortumab vedotin + pembrolizumab versus chemotherapy, is in patients that receive adjuvant immunotherapy and then have a recurrence, and perhaps subsequently randomizing patients to enfortumab + pembrolizumab versus enfortumab alone:
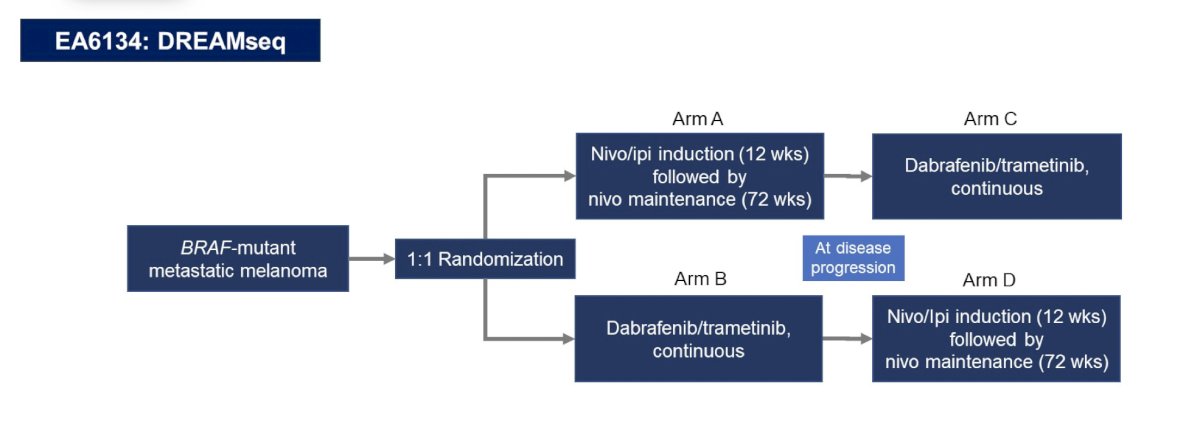
In melanoma, Dr. Pal notes that Dr. Michael Atkins (Georgetown University) states “My lesson from Dreamseq is that if you want to enhance long term survival, you need to give the best immunotherapy first.” The EA6134 DREAMseq trial design is as follows:
Dr. Pal then highlighted that based on the DAD trial presented at ESMO 2023 assessing sacituzumab + enfortumab vedotin, the next step is assessing DAD-IO: enfortumab + pembrolizumab + sacituzumab versus enfortumab vedotin + pembrolizumab followed by sacituzumab in patients with metastatic urothelial carcinoma.
The third take-away point from other disciplines is that rational combinations are the way forward. In the melanoma field, Dr. Pal highlighted Dr. Georgina Long (Melanoma Institute Australia) who states “In melanoma, we have consistently shown that adding checkpoints improves the outcome for patients, including overall survival. We saw this synergist effect with PD1 + CTLA4, and now PD1 + LAG3.” The trial design for the RELATIVITY-047 trial is as follows:
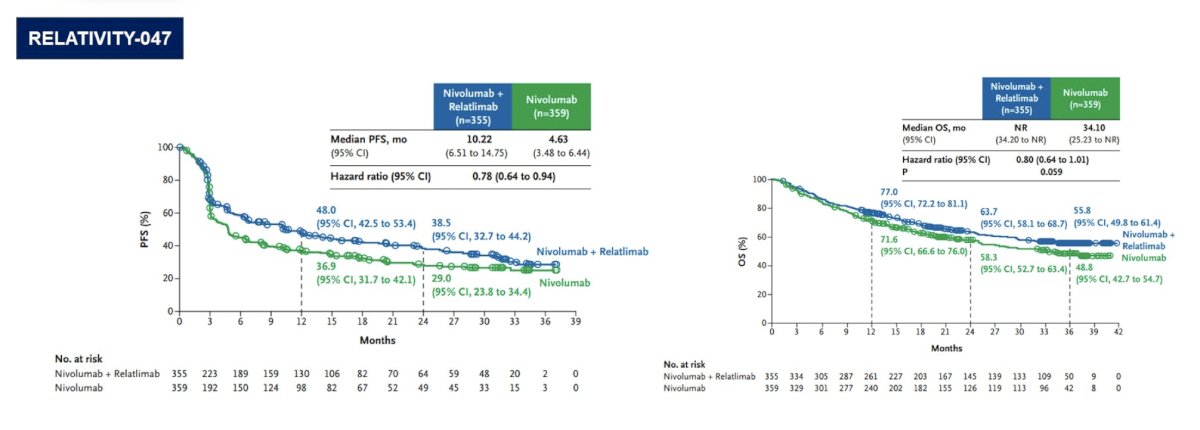
Assessing the progression free and overall survival curves, RELATIVITY-047 showed benefit for nivolumab + relatimab versus nivolumab alone:
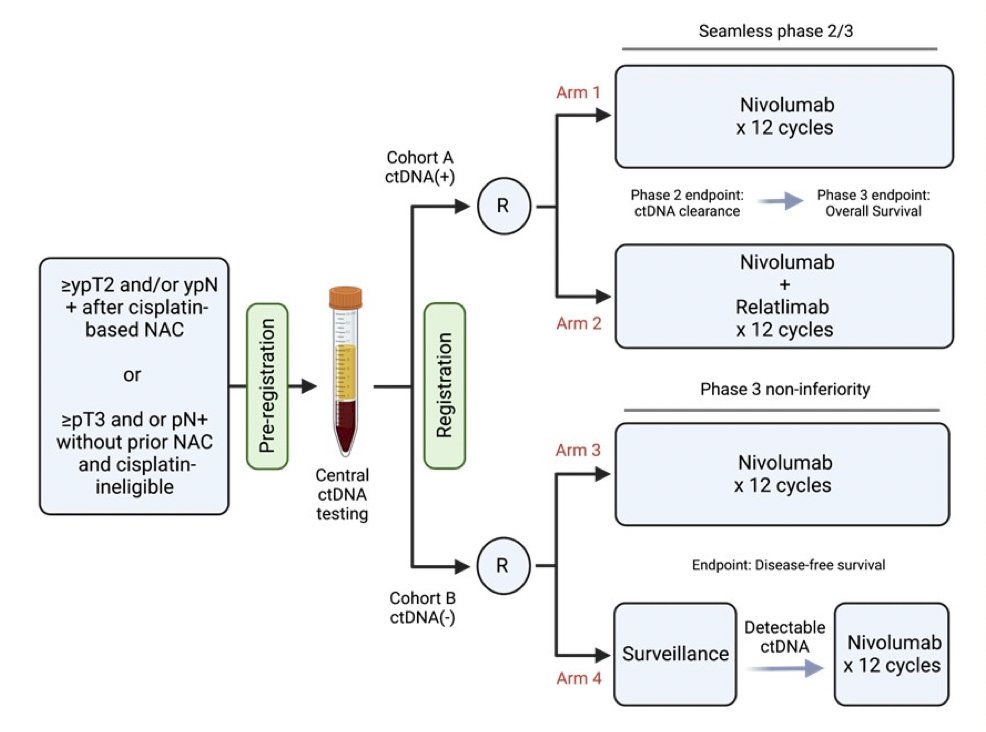
Relating to bladder cancer, Dr. Pal noted the A032103 (MODERN) trial (Matt Galsky – PI) which uses ctDNA as a stratification factor before randomization to one of four arms:
In prostate cancer, Dr. Pal quoted Dr. Neeraj Agarwal (Huntsman Cancer Institute) who states “The success of CONTACT-02 lends itself to pairing the right drugs, in this case cabozantinib and atezolizumab, in the right clinical setting. Rational combinations must be applied in the context of appropriate clinical rationale.” The trial design for CONTACT-02, for which the first results were presented by Dr. Agarwal at GU ASCO 2024, is as follows:
CONTACT-02 reported a median radiographic PFS that was significantly longer with cabozantinib + atezolizumab vs 2nd novel hormonal therapy (control): 6.3 vs 4.2 months; HR 0.65, 95% CI 0.50-0.84, with immature overall survival data:

However, there was a strong OS signal in a prespecified subgroup with liver metastasis (16.4 vs 9.8 months; HR 0.60, 95% CI 0.35- 1.02):

Again, referring back to the DAD-IO assessing enfortumab + pembrolizumab + sacituzumab versus enfortumab vedotin + pembrolizumab followed by sacituzumab, perhaps this trial design should be centered around patients with metastatic urothelial carcinoma with liver metastasis:

The fourth and final take-away point from other disciplines is that we need to keep quality of life at the forefront. Dr. Pal highlighted Dr. Ethan Basch (Lineberger Comprehensive Cancer Center) on Quality of Care stating “Digital symptom monitoring with patient reported outcomes has been shown in randomized trials to catch toxicities early, thereby reducing hospitalization, improving symptom control and quality of life, lengthening time on treatment, and in some cases prolonging overall survival.” The STAR trial design among patients receiving chemotherapy for metastatic breast, lung, GU, and gynecologic cancer at MSKCC is as follows:
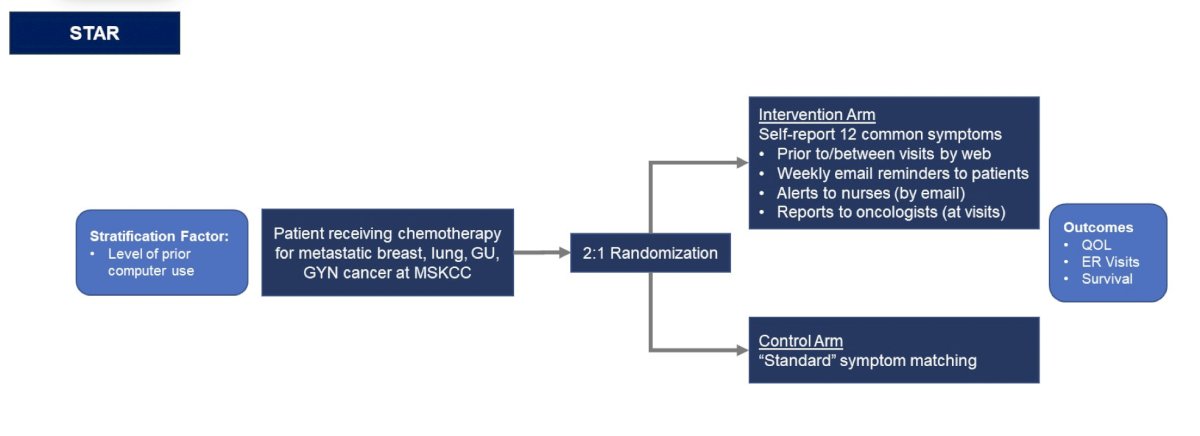
This study showed that patient-reported symptom monitoring was associated with improved overall survival compared to usual care:

Relating to bladder cancer, for the EV-302 trial, among patients that received enfortumab vedotin + pembrolizumab, an adverse event metric whereby physicians and patients decide on adverse events had a lower rate versus traditional assessments, thus perhaps allowing remote monitoring to be incorporated into future trials.
Dr. Pal also highlighted Quality of Life expert Dr. Cristiane Bergerot (CETTRO Cancer Research Hospital) stating “Quality of life is being assessed for contemporary systemic treatments using metrics that were developed decades ago. We need to work with both patients and investigators to update these metrics.” Indeed, some of the tools we are using in metastatic RCC go back 30 years when our current treatments did not exist:
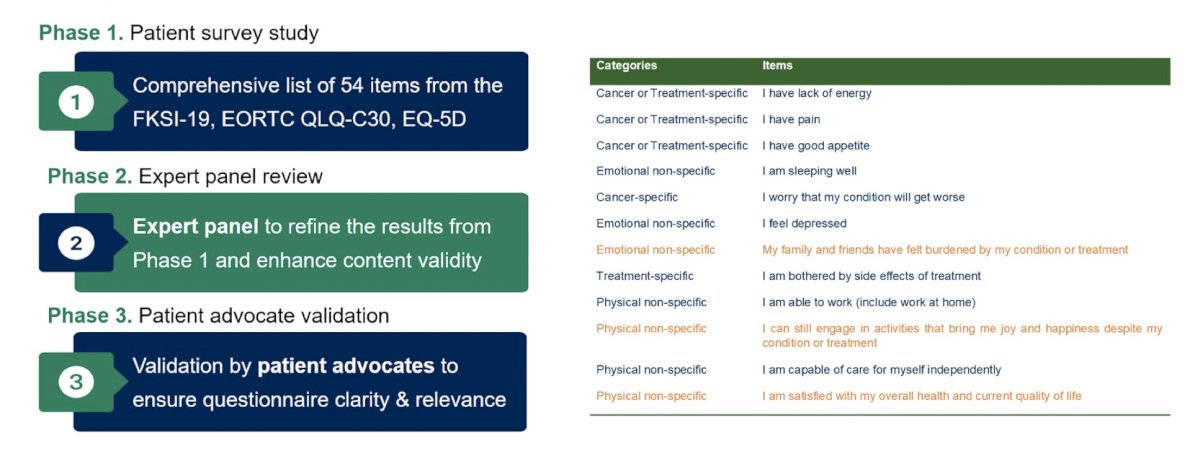
Work from Dr. Bergerot presented at GU ASCO 2024 notes a three phase study (phase 1 - patient survey, phase 2 - expert panel review, phase 3 patient advocate validation) to develop contemporary tools for assessing quality of life among patients with metastatic RCC:
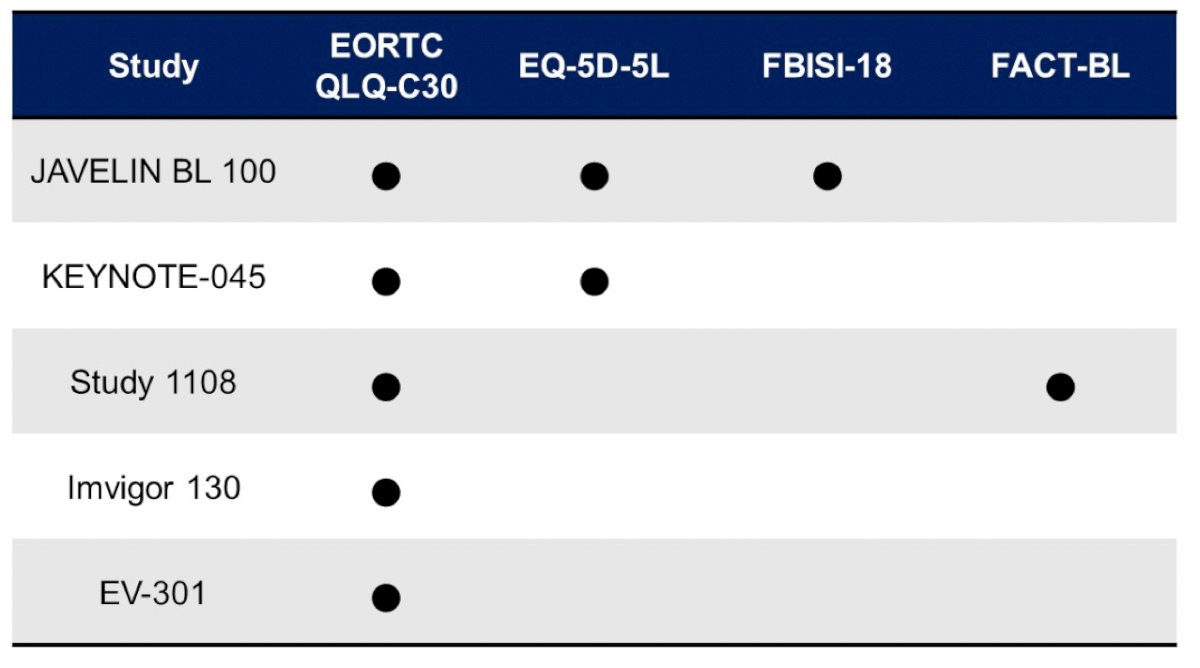
Related to bladder cancer, Dr. Pal emphasized that based on the available tools to date, we need a revised scale that incorporates considerations around contemporary therapies:
Dr. Pal concluded his presentation by discussing what we can learn from other disease sites regarding the implications of checkpoint therapy in urothelial carcinoma by again highlighting the four takeaway points from his multidisciplinary colleagues:
- Adjuvant therapy is good, but neoadjuvant is possibly better
- Optimal sequencing is of paramount importance
- Rational combinations are the way forward
- Keep quality of life at the forefront
Presented by: Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


