(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma oral abstract session. Dr. Max Kates delivered the discussant for the preceding two abstract presentations:
- AMBASSADOR Alliance A031501: Phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma versus observation
- Predicting clinical outcomes in the S1314-COXEN trial using a multimodal deep learning model integrating histopathology, cell types, and gene expression
He noted that these two studies have the potential to act as ‘disrupters’ to the current muscle invasive bladder cancer (MIBC) treatment paradigm. The AI model developed by Dr. Faltas’ lab improves the prediction of treatment response to neoadjuvant chemotherapy and may allow for better selection and more widespread adoption of bladder preservation for complete responders. Dr. Apolo’s study of adjuvant pembrolizumab (AMBASSADOR) is the 2nd adjuvant immune checkpoint inhibitor therapy trial to demonstrate a disease-free survival benefit. This has important implications for adopting a multimodal, multidisciplinary approach for patients with locally advanced bladder cancer.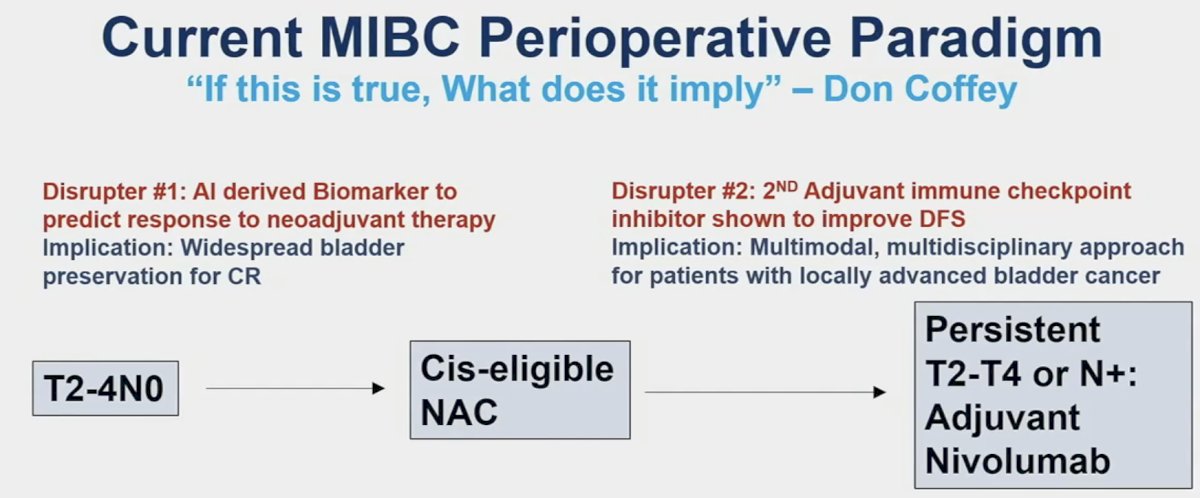
These studies also fit well with the current paradigm shift/evolution that we are witnessing in the MIBC space, whereby we are seeing an increased adoption of bladder preservation across the disease continuum, both for high-risk NMIBC and MIBC.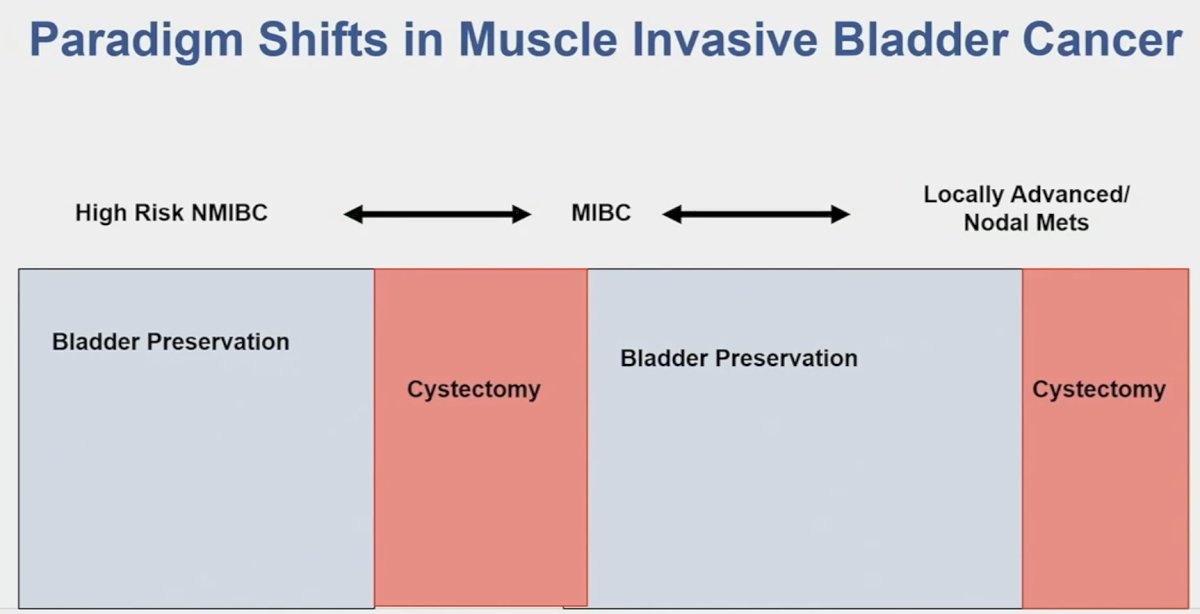
In the COXEN trial, the cohort/data of which was used to derive the AI model by Faltas et al., there was 30.8% complete response rate with neoadjuvant chemotherapy. Importantly, a pathologic complete response was a highly prognostic surrogate endpoint, as seen below, whereby CR patients had excellent long-term survival outcomes.1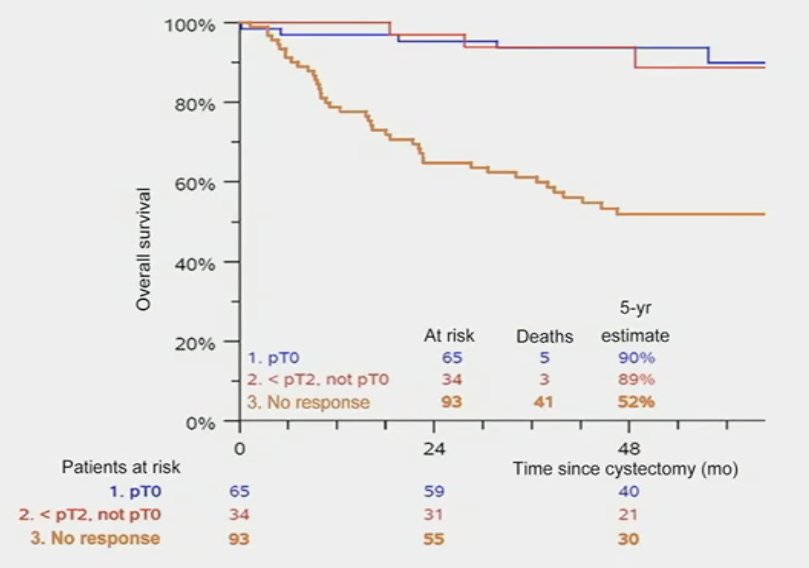
In other words, if we can reliably predict CR, then we can preserve bladders. This has been tested in several studies, including the RETAIN study, whereby patients receiving neoadjuvant ddMVAC with evidence of a complete clinical response and were mutation positive for ATM, RB1, FANCC, or ERCC2 underwent active surveillance (n=26). Of these 26 patients, 69% had disease recurrence on surveillance and 8 underwent a cystectomy. To date, 12 (46%) are metastasis-free with an intact bladder and free from bladder radiation.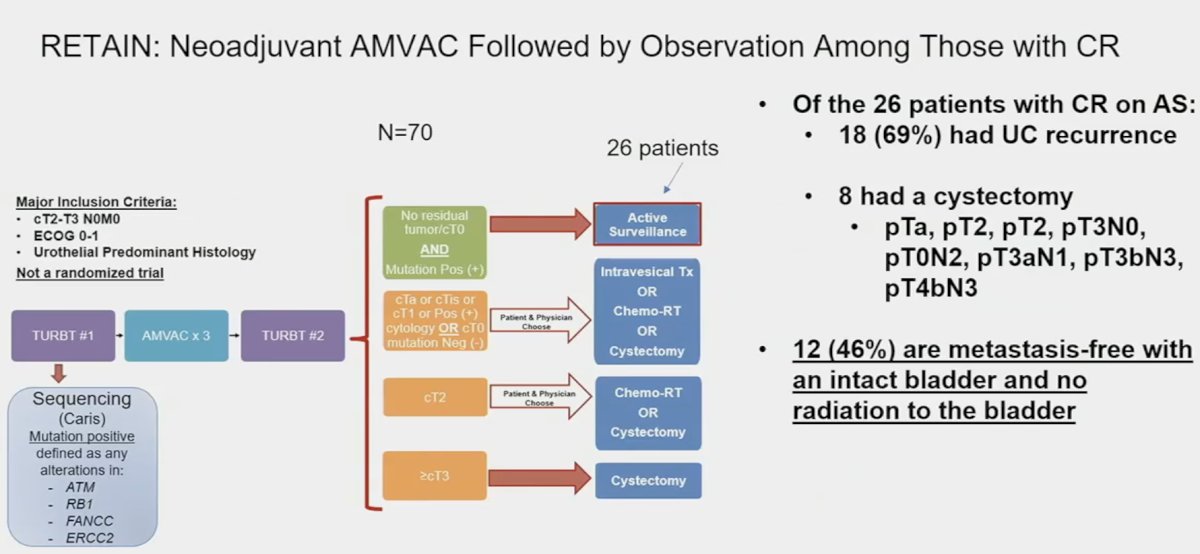
Another phase 2 trial of nivolumab + gemcitabine/cisplatin similarly evaluated outcomes among pathologic complete responders but failed to demonstrate added value to genomic testing.2 As such, additional information and tools to inform potential bladder sparing treatment decisions are sorely needed. This is where Dr. Faltas’ AI model comes into play.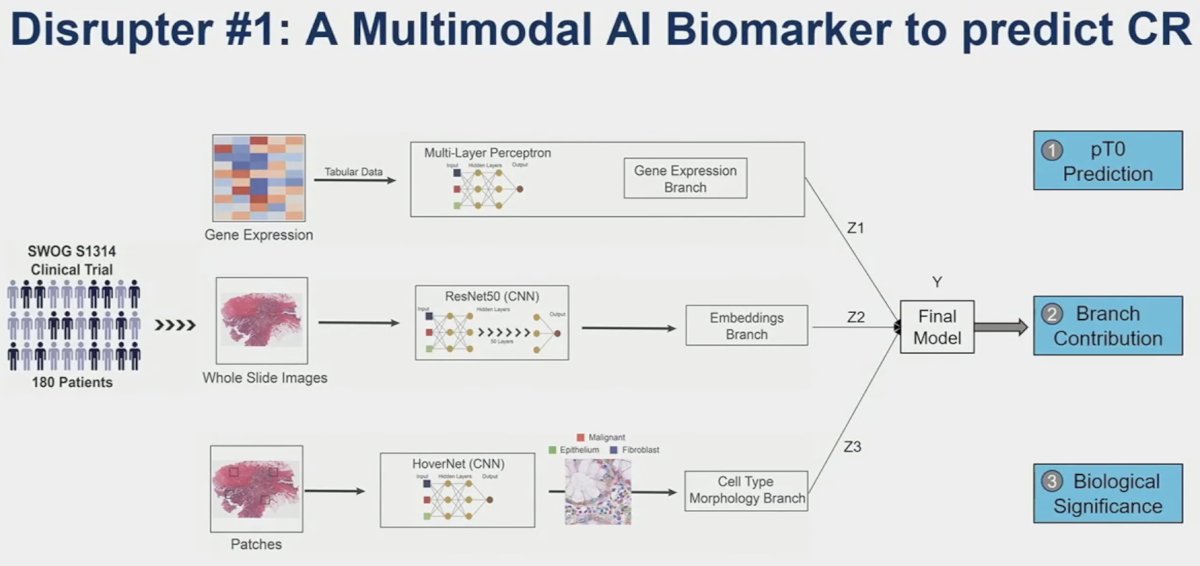
They demonstrated that this multimodal model, combining gene expression patterns, whole slide images, and cell type/morphology patches is able to predict pCR better than any single component alone. This is a valuable tool that can aid clinicians to better predict those with a CR who can retain their bladder.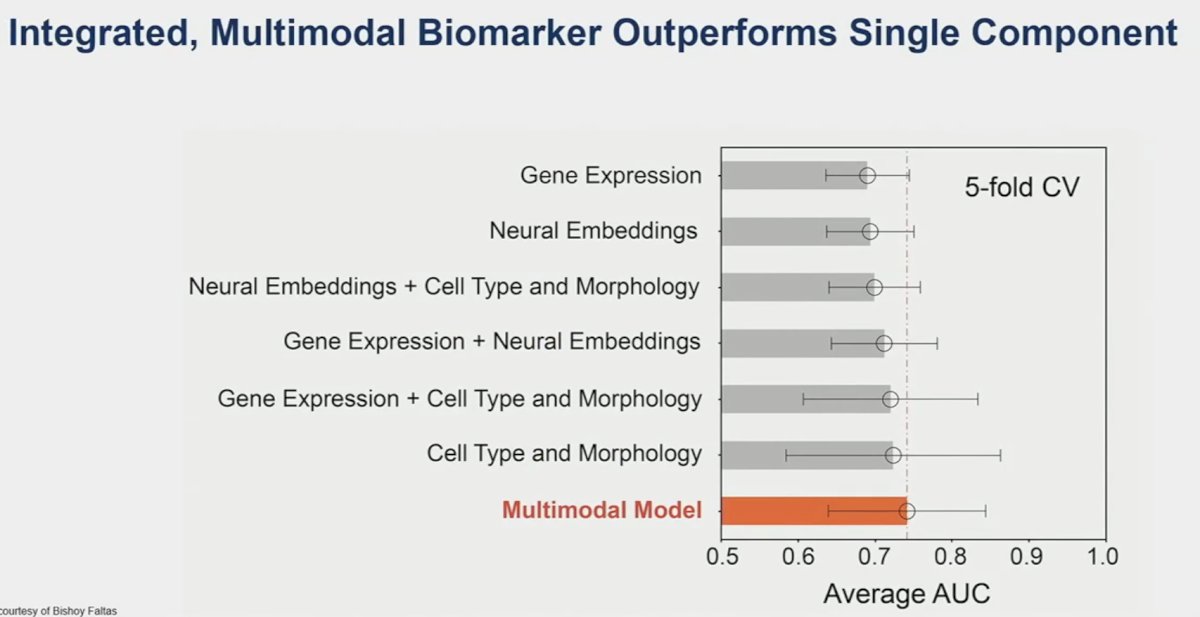
Next, Dr. Kates shifted gears to discuss ‘disrupter #2’: the AMBASSADOR trial. Previous trials in this space have included IMvigor-010 (atezolizumab) and CheckMate-274 (nivolumab).3,4 IMvigor-010 was a negative trial, failing to demonstrate a DFS benefit for atezolizumab in the adjuvant setting. Conversely, CheckMate-274 demonstrated a significant DFS benefit and is now FDA approved for this indication. Now, we have AMBASSADOR as a ‘tiebreaker’ trial, demonstrating a significant DFS benefit. Notably, this was a more heavily pre-treated cohort compared to the previous two trials, with 64% of patients receiving neoadjuvant chemotherapy (64% versus 43-48%). We also note that the median DFS in the pembrolizumab arm (29 months) was significantly longer compared to those observed in the other two active treatment arms (~20 months).
Unanswered question for adjuvant therapy in MIBC remain:
- Do all patients with high-risk features need adjuvant therapy?
- Is one year of pembrolizumab (18 cycles) or nivolumab (24 cycles) necessary for all patients?
- Is pembrolizumab monotherapy the future or is
enfortumab vedotin + pembrolizumab inevitable?
- Currently, there are two trials EV-303 and EV-304 that are evaluating the combination of enfortumab vedotin + pembrolizumab in the MIBC perioperative setting
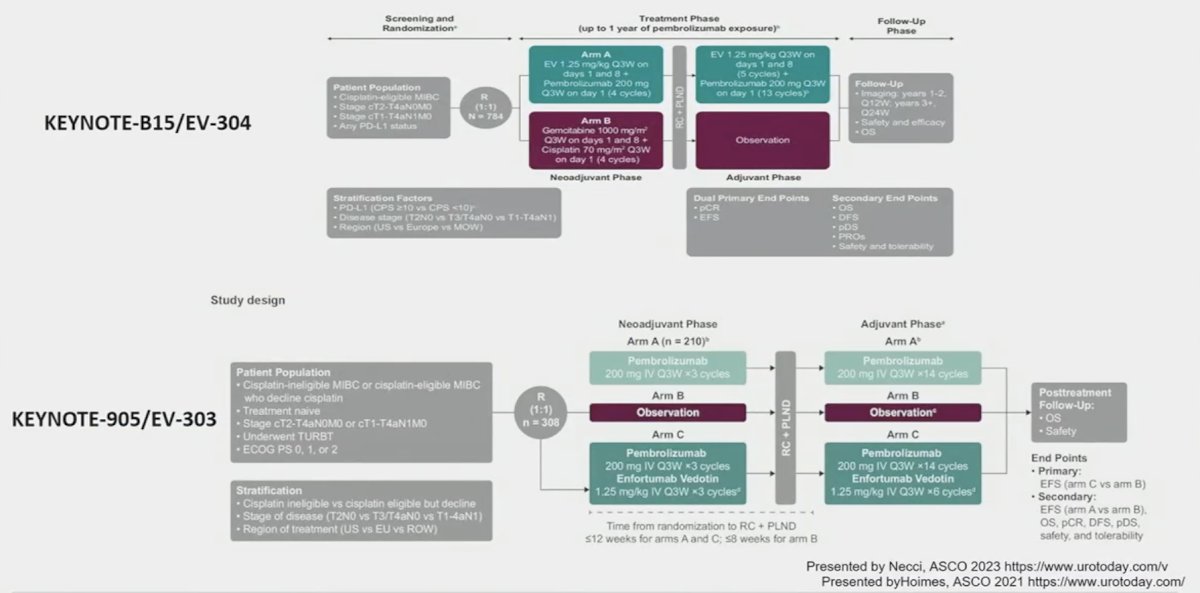
ctDNA may hold promise in this regard with secondary analysis of IMvigor-010 demonstrating that ctDNA holds value as a predictive biomarker to help determine which patients benefit from adjuvant atezolizumab therapy. This evidence is a key rationale for the A032103 (MODERN) trial that is evaluating adjuvant therapy treatment combinations (nivolumab, nivolumab + relatlimab, or surveillance) based on ctDNA status.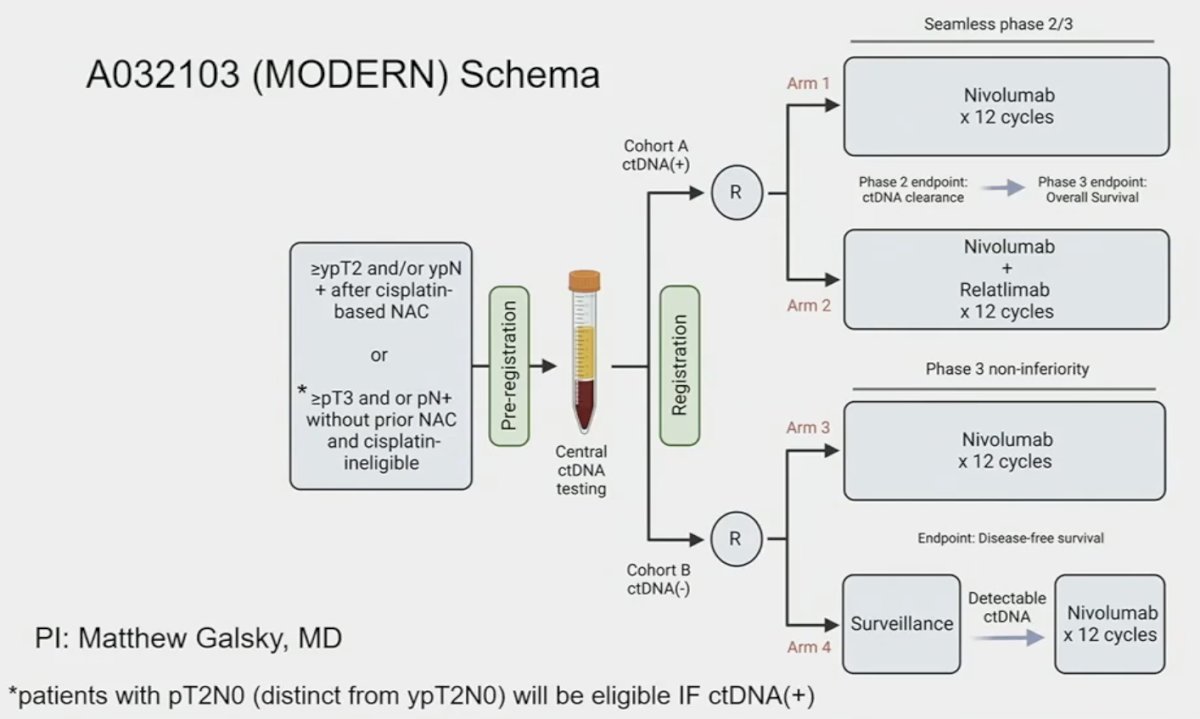
To conclude, Dr. Kates shared his vision of the future paradigm in cis-eligible patients, based on the current evidence and where he sees the field evolving: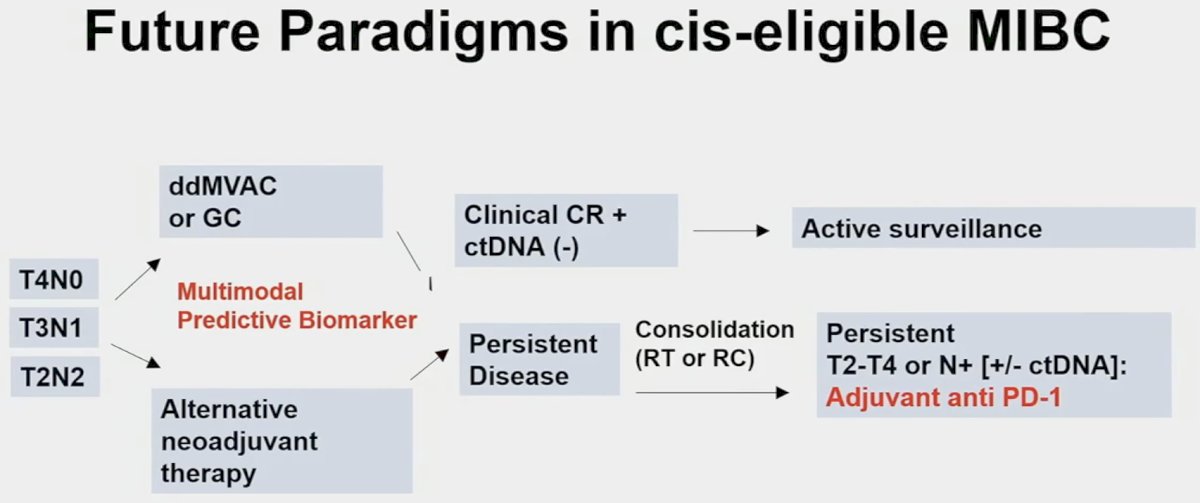
Presented by: Max R. Kates, MD, Associate Professor of Urology and Oncology, Brady Urological Institute, Johns Hopkins Medical Center, Baltimore, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Flaig TW, Tangen CM, Daneshmand S, et al. Long-term Outcomes from a Phase 2 Study of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer (SWOG S1314; NCT02177695). Eur Urol. 2023;84(3):341-347.
- Galsky MD, Daneshmand S, Izadmehr S, et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat Med. 2023;29:2825-2834.
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.


