(UroToday.com) The 2024 GU ASCO annual meeting featured a Keynote Lecture by Dr. Cheryl Lee discussing increasing patient representation in genitourinary malignancy trials. Dr. Lee started her presentation by noting that there are many benefits of clinical trial participation, including that (i) cancers included in clinical trials have the greatest advances in treatment and survival, (ii) trials provide access to innovative therapies that are otherwise unavailable, and (iii) trial participants have better clinical outcomes compared to non-participants. However, there are barriers to clinical trial participation, such as trust in medical research, complexity of participation, and social determinants.
Based on a National Academy 2022 Consensus Report, lack of representation results in several issues with clinical trials:
- It compromises the generalizability of clinical research
- It hinders innovation and new discoveries
- It may compound low accrual and trial failure
- It may undermine trust in clinical research
- It may compound health disparities
Importantly, Dr. Lee notes that there are eight specific groups of individuals that experience cancer health disparities and are underrepresented in clinical trials: (i) belonging to a different ancestry, race, or ethnicity, (ii) low socioeconomic status, (iii) a lack of or having limited health insurance coverage, (iv) certain immigrants, refugees, or asylum seekers, (v) individuals with disabilities, (vi) adolescents and young adults, (vii) the elderly, and (viii) sexual and gender minority communities:
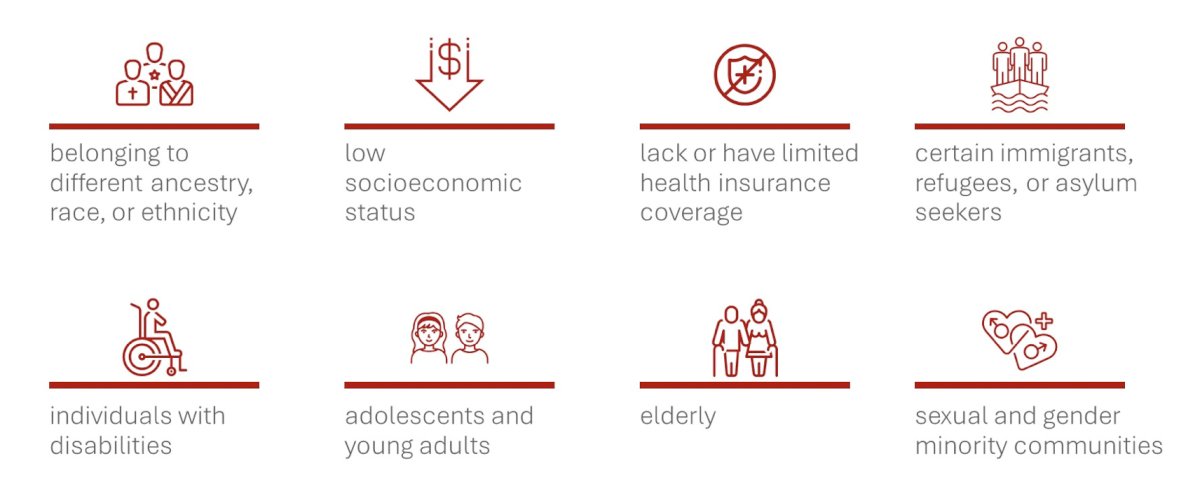
Dr. Lee then highlighted several sobering statistics:
- Individuals living in rural areas have a 17% higher death rate from all cancers combined
- Two-thirds of rural cancer survivors from Appalachia report financial distress
- Black and Hispanic patients with cancer have increased use of financial coping behaviors, such as skipping medications, because they experience adverse financial impact twice as often as White patients
Dr. Lee notes that currently, only 2.5% of patients in clinical trials are black, and only 2.3% are Hispanic:
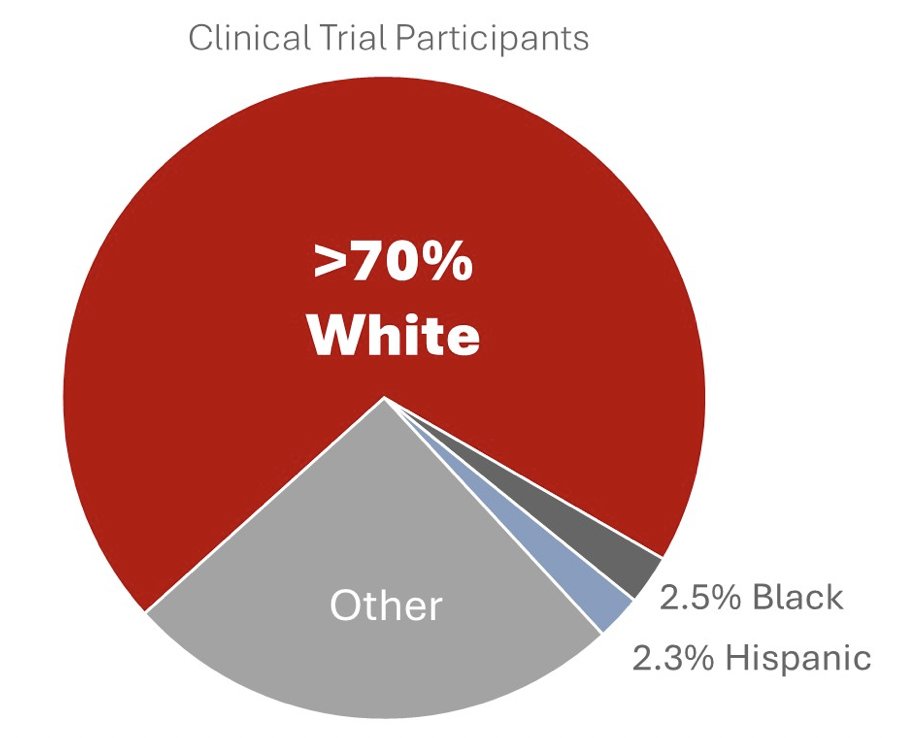
The downstream impact of these statistics is that 77% of tumor samples in The Cancer Genome Atlas are from White populations. Between 2009 and 2019, the FDA approved 81 oral anticancer chemotherapeutics based on data from 142 clinical trials, however only 52% of these trials reported on race and ethnicity. As such, the impact is that we are not serving, not learning, and not gaining impact for the diversity of our real world population that these drugs are being tested in order to improve care.
To discuss advancing care through clinical trials, Dr. Lee started by discussing the current demographic representation. Looking at data from 93 Precision Oncology clinical trials (from various sponsors) among 5,867 patients, White patients are over-represented in clinical trials based on the ratio of the actual number enrolled to the expected number based on the US census. Additionally, Hispanic, Black, and American Indian/Alaska Native are all under-represented:
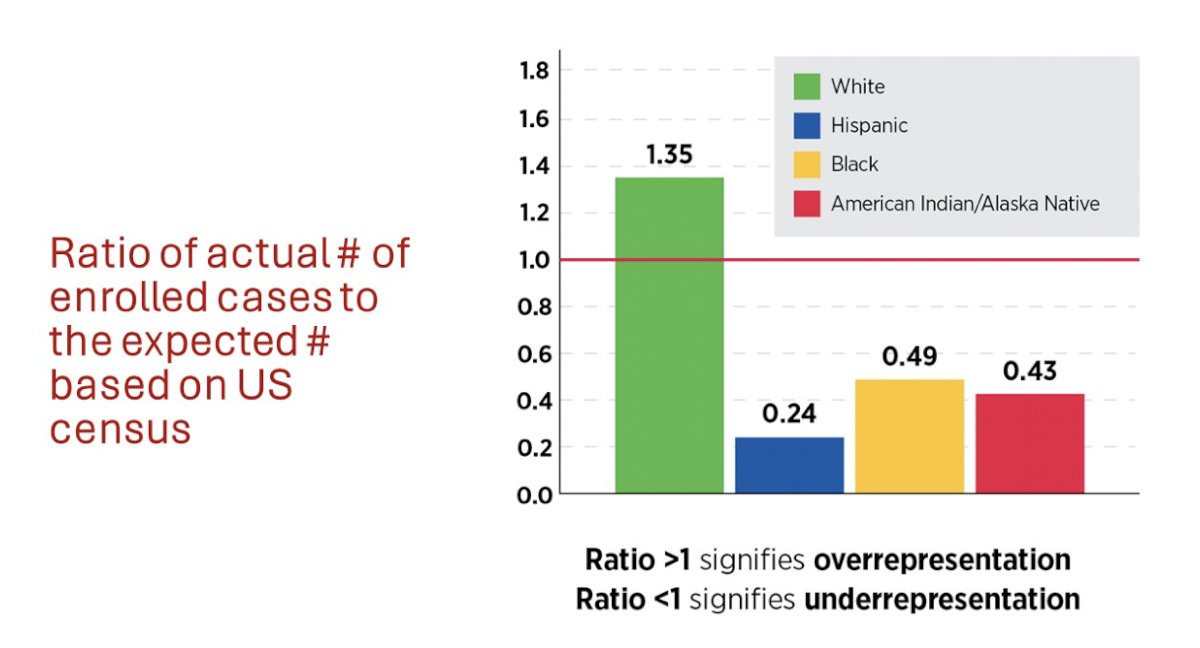
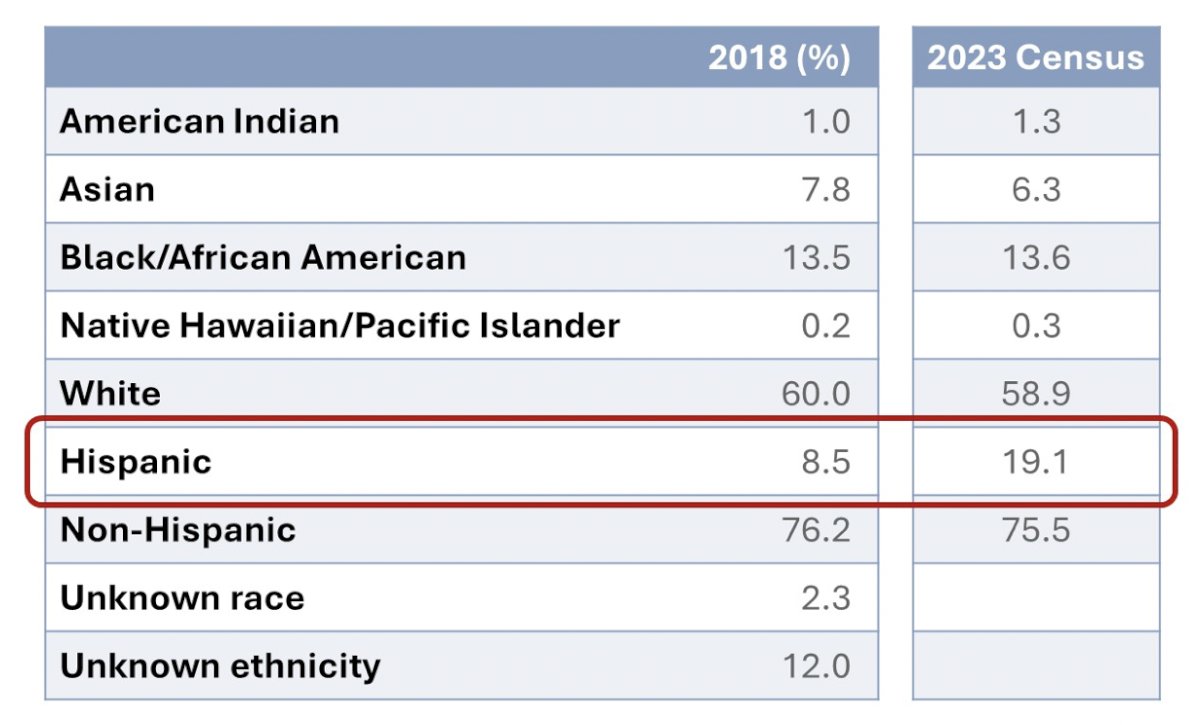
When assessing demographics of participants in trials supported by NIH Centers and Institutes, there is more balance between White and Hispanic participants in relation to the US census, however, Hispanic participants are still woefully underrepresented.
The other issue is that in the case of kidney cancer incidence, there is an increasing trend for American Indian/Alaska Native patients, thus they are further marginalized by underrepresentation in clinical trials:1
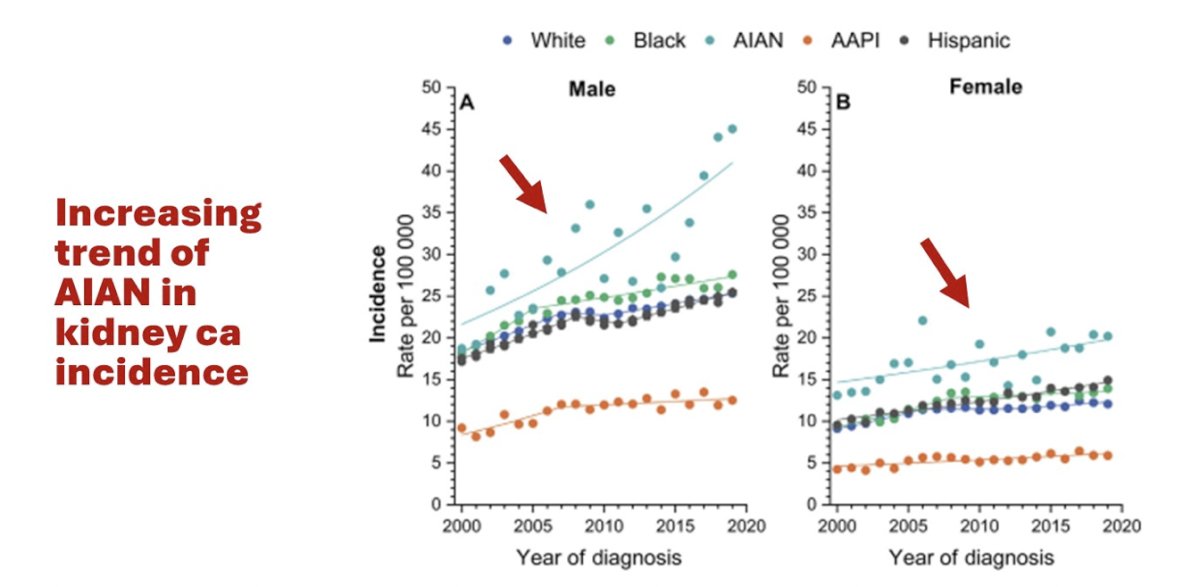
Similar issues and are present in Black men and prostate cancer, given that Black men have the highest incidence of prostate cancer across stage groups:1

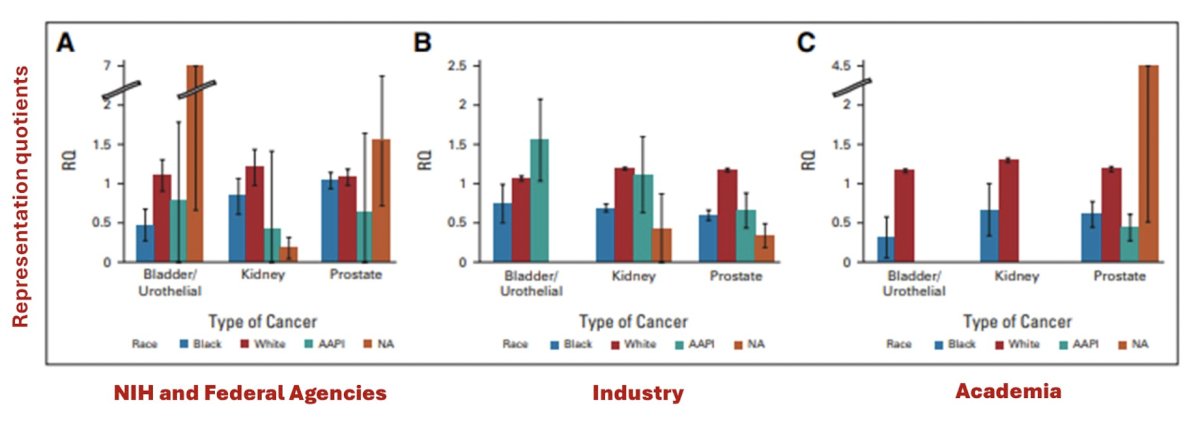
To further highlight this lack of representation of minorities in US prostate cancer trials, work from Owens-Walton and colleagues2 suggests over-representation of white men (RQ 1.15) and an under-representation of Black men (RQ 0.63). This disparity was particularly notable in industry trials (RQ 1.18 for white men vs 0.34 for Black men):
The second aspect of advancing care through clinical trials is addressing GU oncology trial inclusion. Generally, in oncology trials leading to FDA approvals (2008-2018), 63% of trials reported at least one race, 8% documented White/Black/Asian/Hispanic, and 16% reported ethnicity. However, less than 50% of trials leading to these approvals reported on races other than White. Specific to bladder cancer, we recently had the presentation of the practice changing data of EV-302 at ESMO 2023, which showed that in first line metastatic urothelial carcinoma, enfortumab vedotin + pembrolizumab outperformed chemotherapy. However, when looking at the Table 1 baseline characteristics from this trial, the trial only listed White and Asian patients and no other races or ethnicities. On the other hand, the COSMIC-313 trial,3 published in 2023, testing cabozantinib + nivolumab and ipilimumab in intermediate/poor risk advanced RCC had a much more detailed and appropriate breakdown of participants by race, including Asian, Black Native American/Alaska Native, White, multiple races, other, and not reported. In GU oncology, representation in prostate cancer is arguably the most important disease space, particularly for Black men. The ProtecT 15-year outcomes published in 20234 showed that prostate cancer-specific mortality is low regardless of treatment modality, however, the baseline characteristics of this trial show that 98-99% of men in this trial were White. Thus, there is no applicability of this trial to minority patients. However, this matches the population of the recruiting centers who were in the trial age range.
The third aspect of advancing care through clinical trials includes assessing tools to increase representation. At ASCO 2023, abstract #6509 “Strategies to Increase Accrual of Underrepresented Populations in Alliance NCTN Trials” addressed some of these issues:
- Leadership promotion: 20% underrepresented minority accrual goal set by leadership for all Alliance trials
- Funding: funding underrepresented minority junior investigators and underrepresented minority research
- Working Groups: establishment of Outreach and Accrual, AYA, and rural health working groups
- Investigators from Underrepresented Minority Populations: Assign disparities co-chairs to trials to provide input
- Translation Services: Translation of patient facing materials to other languages
- Focus on Sites: Opening studies at sites with high rates of underrepresented minority populations
- Real-Time Monitoring: Monitoring accrual by demographics and site, in real time
- Special Studies: Protocols developed with aims for specific populations
- Protocol Considerations: Make eligibility criteria less burdensome and inclusive
- Operational Strategies: Increasing sample size, losing studies to non underrepresented minority participants
In November 2020, the FDA released a guidance document “Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry” stating that to “further promote and protect public health, it is important that people who are in clinical trials represent the populations most likely to use the potential medial product.” This guidance document made the following suggestions based on enhancement strategies, trial design and methodology, and inclusive trial practices:

Along a similar line of thought, the ASCO-Friends of Cancer Research Joint Research Statement suggests the following changes to make clinical trials more representative and inclusive:
- Have washout periods
- Less stringent rules on concomitant medications
- Allow prior therapies
- Softer laboratory reference ranges/test intervals
- Allow poorer performance status patients
- Allow patients with brain metastases
- Include patients < 18 years of age
- Allow those with HIV infection
- Adjust the inclusion criteria for those with prior and concurrent malignancies
- Adjust the inclusion criteria for those with organ dysfunction (ie. cardiac, liver, and kidney)
Additionally, guidance from the FDA Guidance for Industry document addresses expanding access, adopting inclusive enrollment and retention practices, and reducing participant burden:

Dr. Lee highlighted that from the American Association of Cancer Research’s 2022 Disparities Progress Report, there are several streamlining strategies we can adopt based on lessons learned from the COVID-19 pandemic, including: (i) electronic consent, (ii) telehealth evaluations, (iii) home delivery of drugs, (iv) lab and image center options, (v) greater community-based engagement, and (vi) greater trial accessibility. An additional strategy is Medicare reimbursement for patient navigation strategies (November 2023). Two collaborative biologic initiatives include the NIH’s All of Us project which has enrolled 100,000, including 50% from underrepresented groups, and the AACR Project GENIE, which has sequenced tumors from more than 121,000 patients, including 16,000 (13.4%) from racial and ethnic minorities.
The final discussion point for advancing care through clinical trials is creating evidence-based strategies to increase representation. CUSP2CT (Connecting Underrepresented Populations to Clinical Trials) is taking a multi-level approach to culturally tailor outreach and education interventions with the primary goal of increasing referral/accrual of underrepresented populations to NCI-supported trials. Specific CUSP2CT sites include UCF Health Cancer Center, University of Miami Sylvester Comprehensive Cancer Center, Ohio State University The James Comprehensive Cancer Center, Moffitt Cancer Center, and the ECOG-ACRIN Cancer Research Group. Specific aims of CUSP2CT at Ohio State University, led by Dr. Lee, are to:
- Phase 1: Conduct a baseline assessment of referral patterns and accrual of racial and ethnic minorities to clinical trials
- Phase 2: Implement multi-level intervention focused in 9 counties in the Ohio State University catchment area that directly intervenes on problems identified at each level
- Phase 3: Evaluate the impact of referral (primary outcome) and accrual patterns (secondary outcomes) on clinical trials
These include multi-level strategies at the patient, provider, system, and community level.

Dr. Lee concluded her presentation by discussing increasing patient representation in genitourinary malignancy trials with the following take-home points:
- Broader representation in clinical trials is critical
- Barriers to participation are multifactorial
- National attention and action is growing
- Key strategies exist to improve representation
Finally, as we look forward, additional initiatives listed as follows are important:
- Study partners: global partners, enriching sites, and having DEI co-investigators
- Writing protocols: relax eligibility, reduce burden, and track race and ethnicity
- Recruiting participants: Cancer Center COE, community ad-boards, and patient navigators
- Reporting Data: these protocols must require race and ethnicity reporting, as do abstracts and manuscripts
Presented by: Cheryl T. Lee, MD, The Ohio State University, Columbus, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Lated content: Charting New Paths: Increasing Patient Representation in Genitourinary Malignancy Trials - Cheryl Lee
References:
- Schafer EJ, Jemal A, Wiese D, et al. Disparities and Trends in Genitourinary Cancer Incidence and Mortality in the USA. Eur Urol. 2023 Jul;84(1):117-126.
- Owens-Walton J, Williams C, Rompre-Brodeur A, et al. Minority enrollment in phase II and III Clinical Trials in Urologic Oncology. J Clin Oncol 2022 May 10;40(14):1583-1489.
- Choueiri TK, Powles T, Albiges, et al. Cabozantinib plus Nivolumab and Ipilimumab in Renal Cell Carcinoma. N Engl J Med. 2023 May 11;388(19):1767-1778.
- Hamdy FC, Donovan JL, Lane JA, et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2023 Apr 27;388(17):1547-1558.


