(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the role of immunotherapy in advanced urothelial carcinoma and a presentation by Dr. Peter O'Donnell discussing drug sequencing, pairing, switching, and the role of checkpoint re-challenging. Dr. O’Donnell started his presentation highlighting three patient scenarios to consider:
- Scenario 1: a 73 year old treated with prior gemcitabine/cisplatin two years ago, achieving a complete response, and maintenance avelumab for 1 year, now with recurrent disease to several pelvic and inguinal lymph nodes
- Scenario 2: 78 year old with diabetes mellitus (HgbA1c 6.5%), reliable with metformin, but with an eGFR of 29, with metastatic disease to the lymph nodes and lung, now progressing on pembrolizumab (after 10 months)
- Scenario 3: 63 year old with prior T3N0 disease at cystectomy, eGFR 45, treated with adjuvant nivolumab x 1 year, but developed newly metastatic disease 6 months after nivolumab therapy
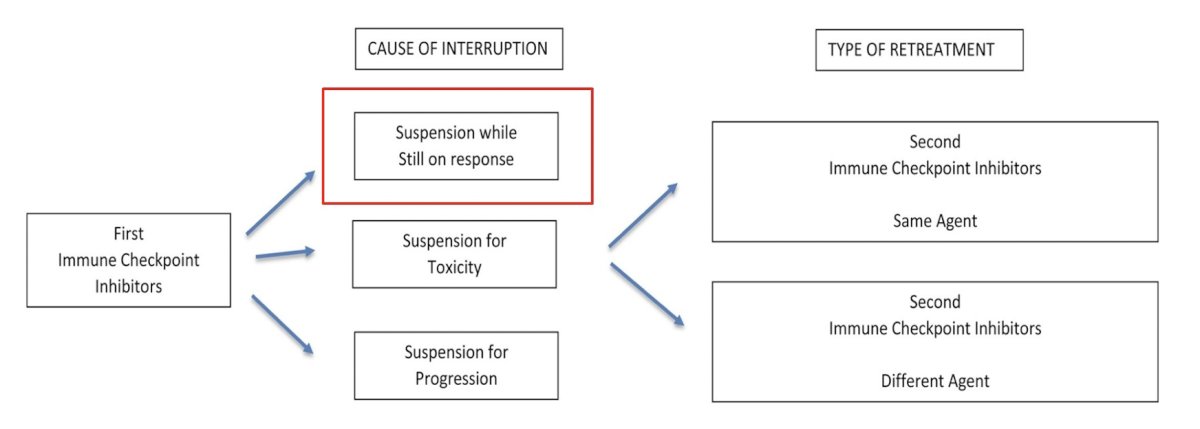
How do we determine whether an I/O-treated patient should be re-challenged? Dr. O’Donnell notes that it is important to understand the cause of the interruption and the type of proposed re-treatment:
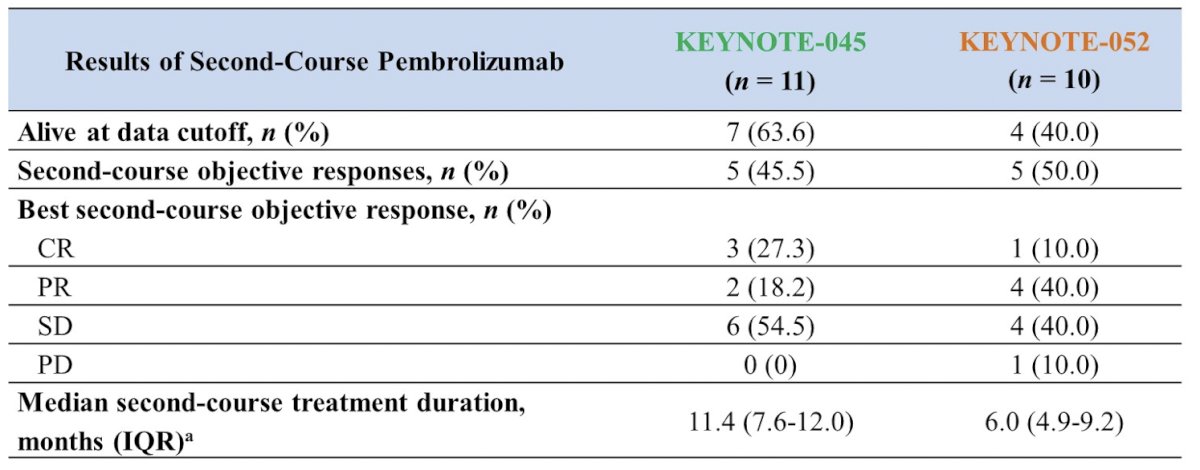
In the setting of re-challenge after prior I/O benefit, there is data from KEYNOTE-0451 (platinum-refractory setting) and KEYNOTE-0522 (treatment naïve setting). Patients that had 2 years of pembrolizumab with stable disease or better, or patients that had 6 months of pembrolizumab with a complete response who subsequently had disease progression after discontinuing I/O were eligible for up to 1 year pembrolizumab re-treatment if no intervening anticancer therapy was administered after the I/O course.3 Among 11 patients in the KEYNOTE-045 trial (5 complete responses, 5 partial responses, 1 stable disease during the first course of I/O) they had a median time off I/O of 7.7 months (IQR 3.6-16.3), and a median duration of second I/O of 11.4 months (IQR 7.6-12.0). Among 10 patients in the KEYNOTE-052 trial (6 complete responses, 4 partial responses during the first course of I/O) they had a median time off I/O of 13.0 months (IQR 9.2-16.6), and a median duration of second I/O of 6.0 months (IQR 4.9-9.2). The second course objective response rate was 45.5% in KEYNOTE-045 and 50.0% in KEYNOTE-052:
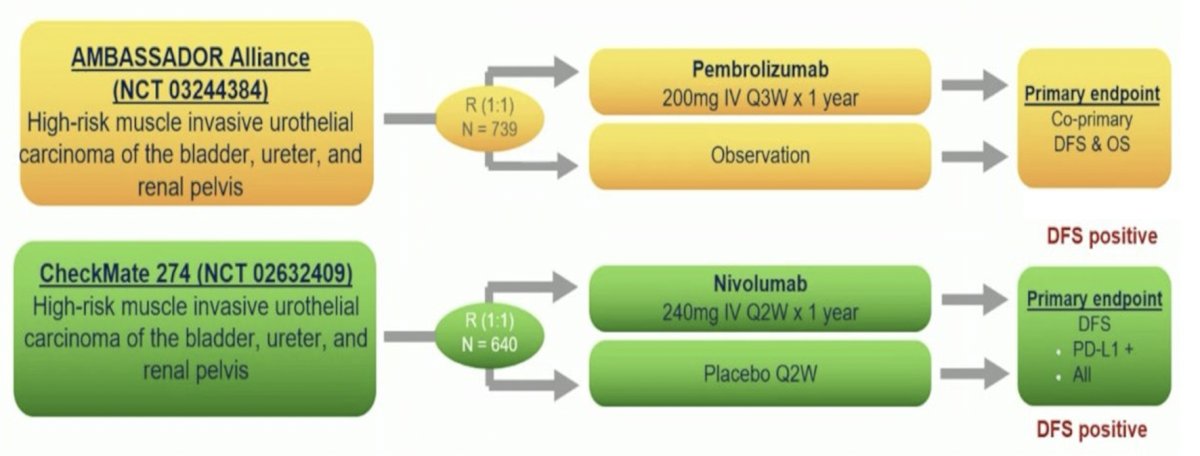
Dr. O’Donnell notes that re-challenge questions are more complex in the situation where immunotherapy was used in the adjuvant setting. There are two disease free survival positive I/O adjuvant trials, including the CheckMate 274 trial and the recently presented at GU ASCO 2024 AMBASSADOR trial:
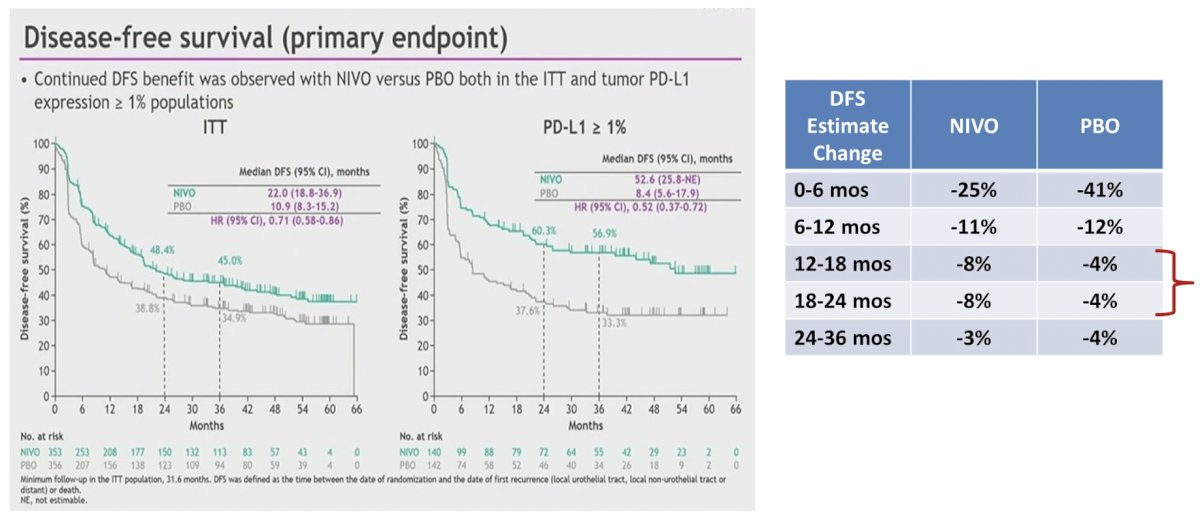
The key question is: how do we determine whether acquired “I/O resistance” has occurred? Also, did immunotherapy resistance develop, or did the I/O effect simply “wear off” after we stopped the drug? We know that the half life of pembrolizumab and nivolumab is ~22-25 days based on pharmacokinetic data. But, what time period since the last I/O is the cutoff to declare “I/O resistance”? Dr. O’Donnell suggests that most proposed guidelines have adopted 6 months in the metastatic setting. Of note, there may be a subpopulation of patients who progress shortly after I/O discontinuation based on the CheckMate 274 disease free survival curves, and thus may have benefited from a continued I/O dose:
Additional considerations for the cause of interruptions are if there was suspension for toxicity or for progression. If there is consideration of switching I/O agents after the development of I/O progression, there may be limited utility and several unanswered questions in this setting:
- Where is the metastatic progression?
- Are there new lesions, or simply growth of existing lesions?
- Can we use radiation to control oligo progression, and continue (or switch) I/O monotherapy?
- Do we introduce a short course of cytotoxic therapy and then resume maintenance I/O?
Among 25 patients in a retrospective analysis, 39% had a complete/partial response as best response to the first course of therapy, 44% had progressive disease as best response to first course of therapy, and 19/25 eventually progressed on first I/O (4/25 stopped the first I/O due to toxicity). Most patients (21/25) were re-challenged with a different immune checkpoint inhibitor than the one initially administered (half switched ‘class’). Overall, there was a 13% response rate on rechallenge (partial + complete response).
Another important question is whether patients who progress on adjuvant or maintenance I/O receive enfortumab vedotin alone or in combination with pembrolizumab? According to Dr. O’Donnell, the answer likely comes from whether or not we believe there is combined activity of enfortumab vedotin + pembrolizumab that has an ‘additive’ or ‘synergistic’ effect. If we look at the first line EV-302 data with a 68% response rate, at first glance it would appear that on their own (based on monotherapy data), there is a contribution of 45% from enfortumab vedotin and 30% from pembrolizumab:

However, Dr. O’Donnell makes the argument that clinically, there is likely some overlap between these two entities and there is likely an unknown amount of this response rate that is secondary to a synergy between enfortumab vedotin and pembrolizumab:

Dr. O’Donnell notes that there likely is a case for using the combination of enfortumab vedotin + pembrolizumab in a patient that has previously received I/O therapy. Given the impressive progression free survival data from EV-302, but also knowing that long-term outcomes from EV-103 Cohort A have a long tail on the curve, perhaps we are actually curing half of these patients:
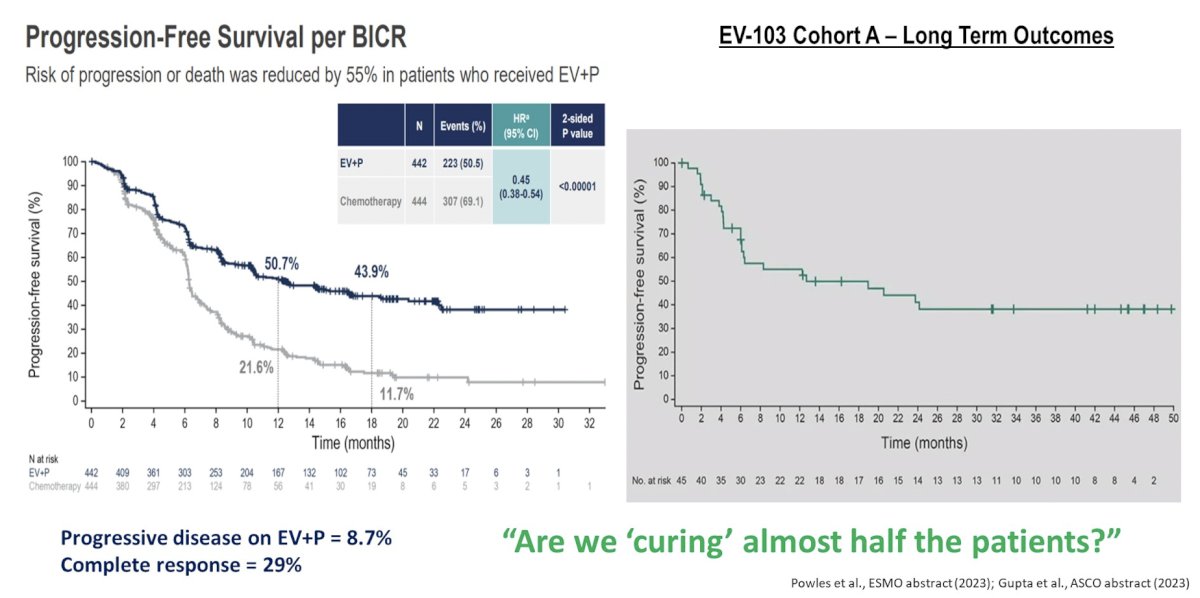
Furthermore, Dr. O’Donnell hopes that enfortumab vedotin + pembrolizumab will be given in our highly comorbid/frail patients in the front line, when historically they may have just received monotherapy.
Dr. O’Donnell emphasized that the immunotherapy “dance partner” likely matters:
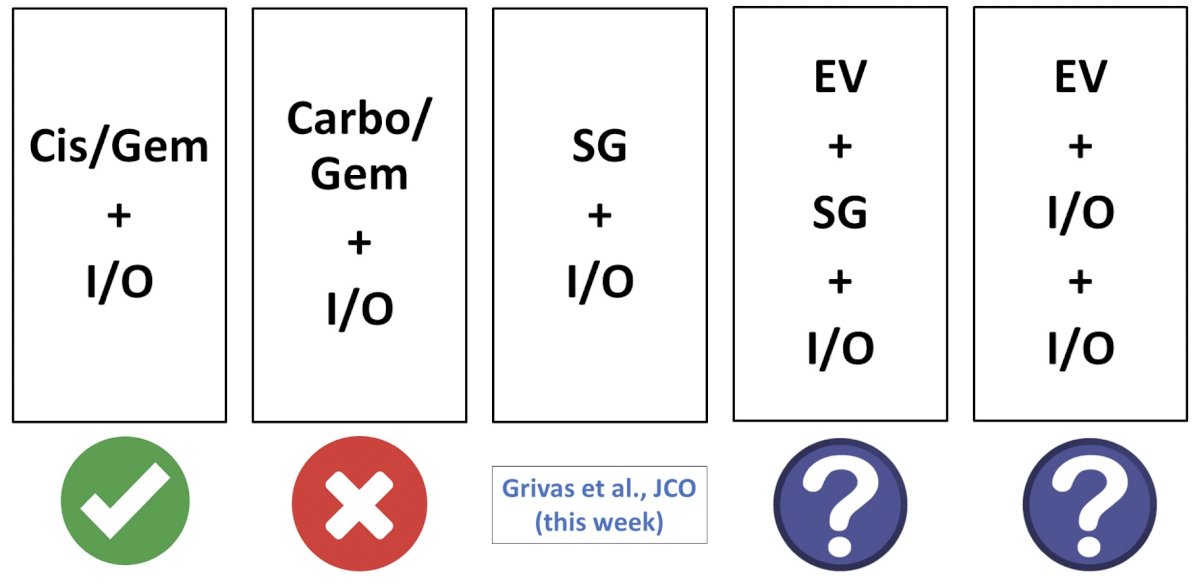
Before concluding his presentation, Dr. O’Donnell referred back to the 3 patient scenarios he used to begin the presentation, noting that a provocative argument could/should be made for considering enfortumab vedotin + pembrolizumab in all of these situations.
Dr. O’Donnell concluded his presentation by discussing drug sequencing, pairing, switching, and the role of checkpoint re-challenging with the following take-home points:
- Immunotherapy agents now represent a foundational component of the disease-altering approaches in advanced urothelial cancer, including all approaches in the first line setting
- Disease progression during I/O monotherapy likely deserves different consideration than progression after prior I/O treatment
- For patients who progress after I/O when it is electively discontinued (6 months) may have a reasonable argument for I/O retreatment based on PK/PD measures and observational data
- Efficacy of enfortumab vedotin + pembrolizumab in prior I/O treated patients deserves investigation. Synergy arguments may support its use, even in patients progressing on prior I/O
- New combinations integrating I/O represent the future
Finally, Dr. O’Donnell re-highlighted the transformative moment in the treatment of metastatic urothelial cancer, the results of the EV-302 trial of enfortumab vedotin + pembrolizumab leading to a >31 month survival benefit in the first line setting:

Presented by: Peter H. O'Donnell, MD, University of Chicago, Chicago, IL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-1492.
- Balar AV, Castellano DE, Grivas P, et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: Results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol. 2023 Mar;34(3):289-299.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.


