(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a session addressing the shortage of drugs for urothelial carcinoma patients and, specifically, why practitioners struggle to get patients what they need and what can we do about this. Dr. Elizabeth Guancial discussed potential approaches to the ongoing platinum shortage from a community oncologist’s perspective.
This has been a challenging year in pharmacy. Shortages have dominated the headlines in 2023. Dr. Guancial noted that since the start of her medical career, shortages have always been an assumed part of life that clinicians have dealt with and moved on. But this year, this shortage has taken on a totally new meaning and significance. So why was this year so different? How did the supply crisis unfold? What was the real-time clinical response? And what are some lessons that can be learned for the future?
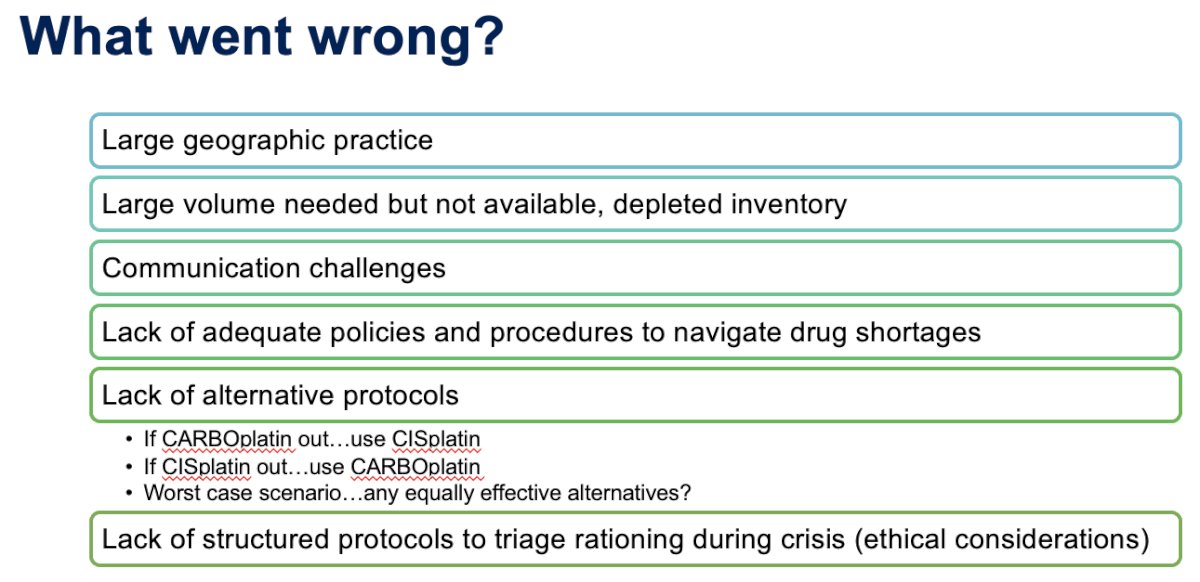
Drawing on her own experiences, she noted that she is one more than 250 oncologists in a large community oncology practice in Florida. She estimated that approximately 550 patients receive carboplatin weekly, and 260 patients receive cisplatin weekly. On March 27, 2023, a national shortage of carboplatin was announced, with no duration of shortage revealed. Dr. Guancial’s practice responded by rounding all carboplatin doses down by 5 to 10%, except when carboplatin was administered with curative intent and in the neoadjuvant or adjuvant setting. Physicians were advised to consider proactively switching eligible patients to cisplatin. Ten days later, on April 7, 2023, with no shortage end in sight, Dr. Guancial’s practice proceeded to round down all patients’ carboplatin doses by 5 – 10%, irrespective of whether planned treatment was curative or palliative.
One month from the original announcement (April 28, 2023), carboplatin was unavailable through all sources. There was only one week's supply of carboplatin remaining. Physicians were asked to develop patient communication and scheduling strategies (e.g., administer chemotherapy cycles every 4 to 5 weeks, as opposed to every 3 weeks, or forego neoadjuvant chemotherapy in favor of upfront cystectomy). Additionally, there was only a 3-week supply of cisplatin remaining. The pharmacy team began to round down all cisplatin doses by 5 – 10%.
On May 3rd, 2023, Dr. Guanical’s practice developed disease-specific recommendations for non-platinum alternatives. For example, enfortumab vedotin + pembrolizumab was identified as an alternative. At that time, the results of the EV-302 trial had not been presented yet at ESMO 2023, and there was no regulatory approval available. Dr. Guancial acknowledged the role of the insurance companies, with most, if not all, approving this ‘off label’ combination at the time after many thorough peer-to-peer reviews.

Two months later, on May 25, 2023, carboplatin and cisplatin were limited to patients with curative intent treatment. Nurse managers worked directly with physicians to identify patients receiving curative-intent carbo/cisplatin. Dr. Guancial noted that this was a testament to the earlier, proactive efforts, otherwise none of this would have been possible. Furthermore, the pharmacy proceeded to operationalize patient-specific supply distributions. But this still meant that many patients were not able to receive the recommended treatment, an unfortunate outcome that required many discussions about goals of care, particularly for those receiving it in the palliative setting.
On June 15, 2023, the first cisplatin delivery from China arrived. Three weeks later on July 7, 2023, carboplatin supply began replenishing. As such, carboplatin could be used for the following indications:
- All curative intent patients
- All patients with gynecologic malignancies, regardless of intent
- All patients with small cell lung cancer, regardless of intent
Cisplatin could be used for the following indications:
- Curative intent only
At this point, clinical trials with platinum-based therapy could re-open enrollment.
On July 24, 2023, all restrictions for cisplatin were removed. Carboplatin restrictions remained in place. Finally, on August 4, 2023, all restrictions were removed for carboplatin, as well.
What was the impact of these drug shortages?

What went wrong?
As far as the utilization of clinical resources, a number of national organizations, including ASCO, have endorsed many of the strategies used in Dr. Guancial’s clinic:
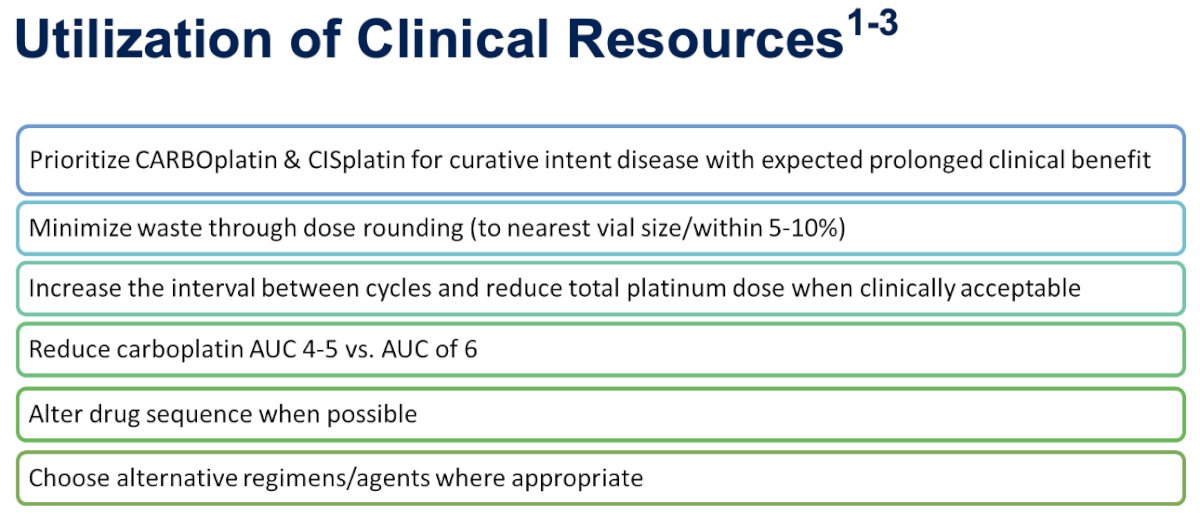
Dr. Guancial concluded that the sooner practices are notified about pending shortages, the sooner they can implement conservation strategies. Success navigating these shortages depends on collaboration between pharmacists and oncologists and clear communication between clinicians and patients.
Presented by: Elizabeth A. Guancial, MD, Oncologist and Hematologist, Florida Cancer Specialists & Research Institute, Sarasota County, FL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


