(UroToday.com) The 2024 GU ASCO annual meeting featured an oral abstract renal cell carcinoma session and a presentation by Dr. Robert Motzer discussing results from Part B of the phase 3 CheckMate 914 trial assessing adjuvant nivolumab monotherapy vs placebo for localized renal cell carcinoma at high risk of relapse after nephrectomy.
CheckMate 914 is a phase 3, randomized, double-blind, multicenter, two-part trial evaluating adjuvant nivolumab + ipilimumab vs placebo (Part A) or adjuvant nivolumab monotherapy vs placebo (and nivolumab monotherapy vs nivolumab + ipilimumab to assess the contribution of components; Part B) designed in sequence in mutually exclusive patients with localized RCC at high risk of post-nephrectomy relapse. Results from Part A at a median follow-up of 37.0 months (range, 15.4–58.0) showed no disease-free survival benefit for adjuvant nivolumab + ipilimumab vs placebo in the overall study population.1 At GU ASCO 2024, Dr. Motzer presented the primary analyses for Part B of the trial.
Patients eligible for CheckMate 914 included those undergoing radical or partial nephrectomy between 4 and 12 weeks before randomization with negative surgical margins with predominantly clear cell histology (with or without sarcomatoid features), pathological TNM stage T2a (grade 3/4) N0M0, T2b-T4 (any grade) N0M0, or any T (any grade) N1M0, and no evidence of residual disease or distant metastases (M0). Patients in Part B were randomized 2:1:1 to nivolumab (240 mg every 2 weeks × 12) + placebo vs nivolumab (240 mg every 2 weeks × 12) + ipilimumab (1 mg/kg every 6 weeks × 4) vs placebo, and stratified by pathological TNM stage and type of nephrectomy. Treatment was planned for 24 weeks (approximately 5.5 months) or until disease recurrence/unacceptable toxicity. The trial design for CheckMate 914 Part B is as follows: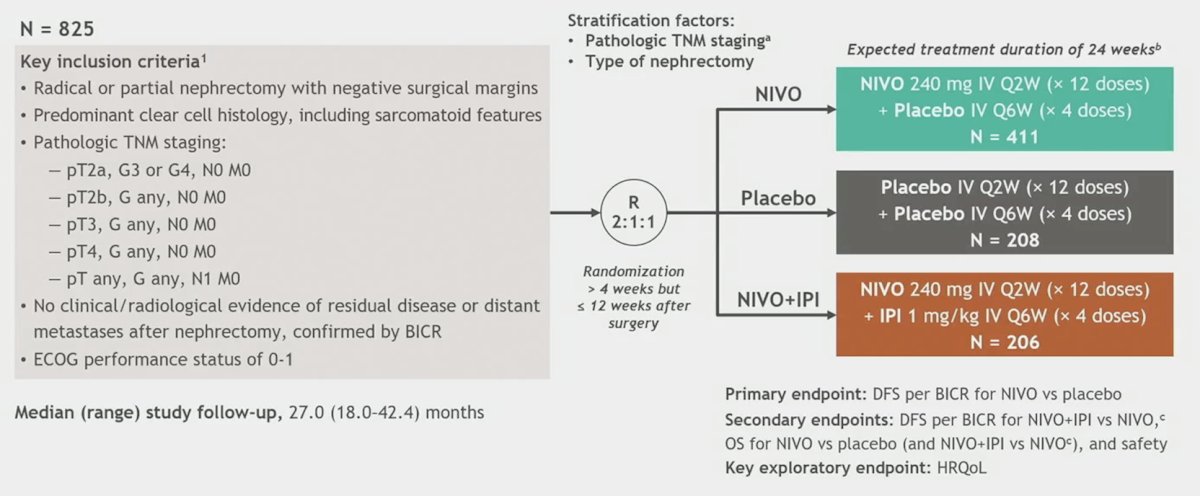
The primary endpoint for Part B is disease-free survival per blinded independent central review (BICR) for nivolumab monotherapy vs placebo. Secondary endpoints include safety of nivolumab monotherapy. From a statistical standpoint, Part B of CheckMate 914 was added by protocol amendment and designed to complement Part A by (i) allowing assessment of adjuvant nivolumab monotherapy versus placebo, and (ii) facilitating the contribution of component analysis. Sample sizes for Part B were driven by the primary endpoint of disease free survival for nivolumab versus placebo, and after 18.0 months of follow-up, the target of 149 disease free survival events had occurred. This provided 60% power to detect an HR of 0.68 at an alpha of 0.05 (2-sided) for the primary endpoint of disease free survival in approximately 600 patients randomized to the nivolumab and placebo arms. The disease free survival was estimated by the Kaplan Meier method and all data reported are based on the clinical data cutoff date of September 28, 2023.
In total, 619 patients were randomized to nivolumab monotherapy (n = 411) or placebo (n = 208). The following highlights select baseline characteristics: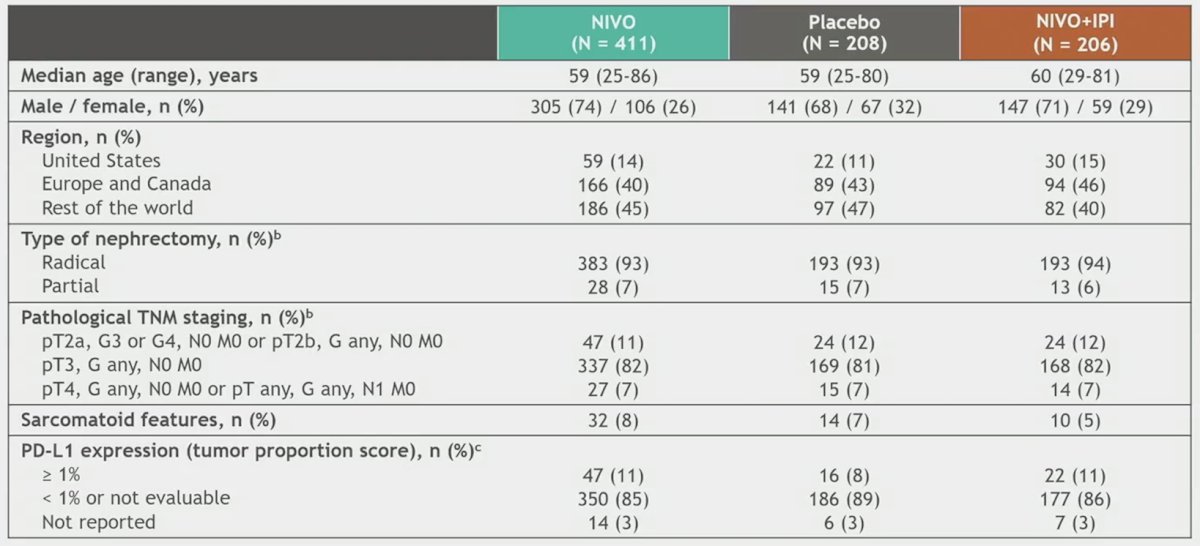
With 27.0 months median study follow-up (range, 18.0–42.4), the primary efficacy endpoint of disease-free survival per BICR with nivolumab monotherapy vs placebo was not met (HR 0.87, 95% CI 0.62–1.21; p = 0.3962):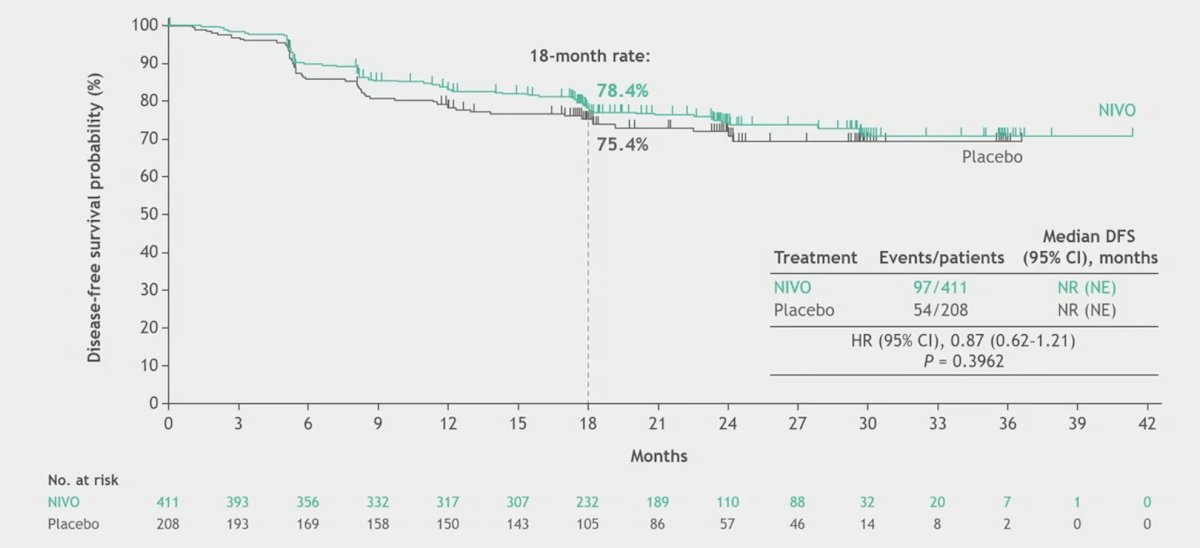
Additionally, the median disease-free survival was not reached in either arm: disease-free survival probabilities were 83.3% vs 78.2% (at 12 months) and 78.4% vs 75.4% (at 18 months), respectively. When assessing select subgroups, Dr. Motzer notes that there may be a signal for benefit in those with sarcomatoid features, PD-L1 expression >= 1%, and those with anemia: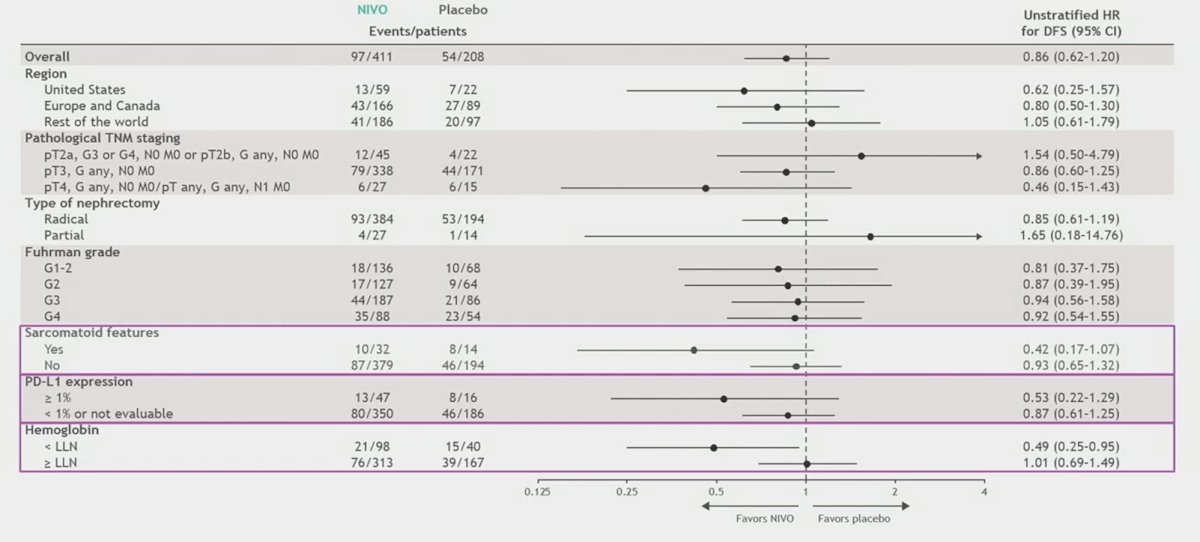
The hazard ratio for disease-free survival per investigator was 0.80 (95% CI, 0.58–1.12; p = 0.1936):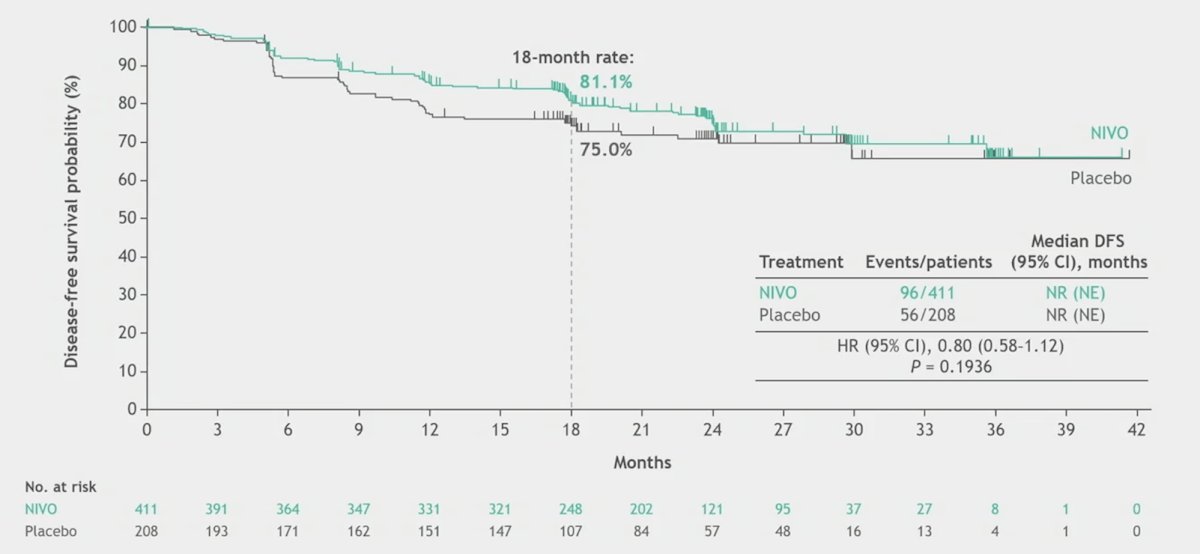
The following shows disease free survival for parts A and B, however, due to the outcomes of CheckMate 914, a contribution of components analysis is no longer relevant: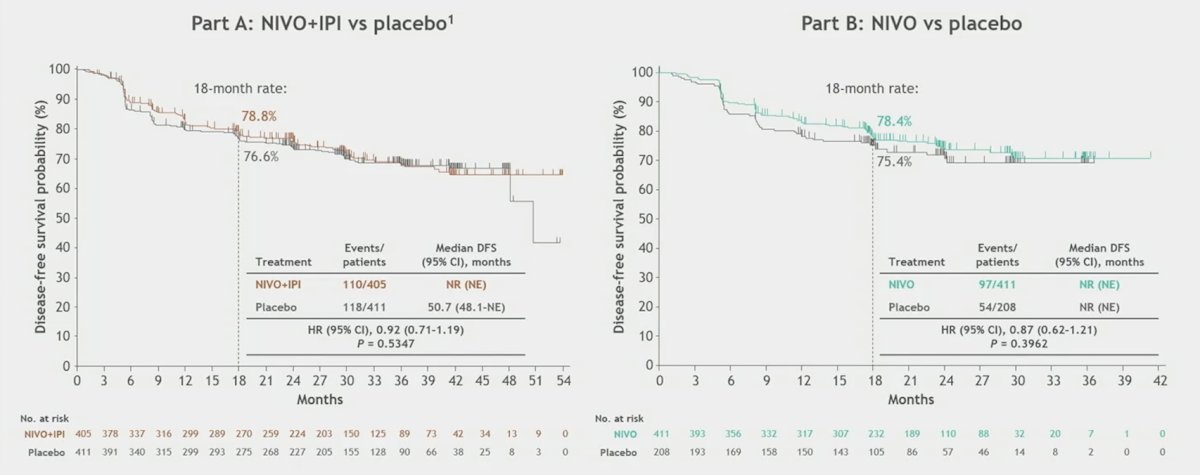
Median treatment duration was 5.1 (Q1, Q3: 5.1, 5.3) months with nivolumab monotherapy and 5.1 (Q1, Q3: 5.1, 5.2) months with placebo. Any-grade treatment-related adverse events were reported in 72.5% vs 51.7% of patients treated with nivolumab monotherapy (n = 408) vs placebo (n = 207), and grade 3–4 treatment-related adverse events were reported in 8.8% vs 1.9%, respectively. Any-grade treatment-related adverse events led to discontinuation in 9.6% and 1.0% of patients in the nivolumab monotherapy and placebo arms, respectively: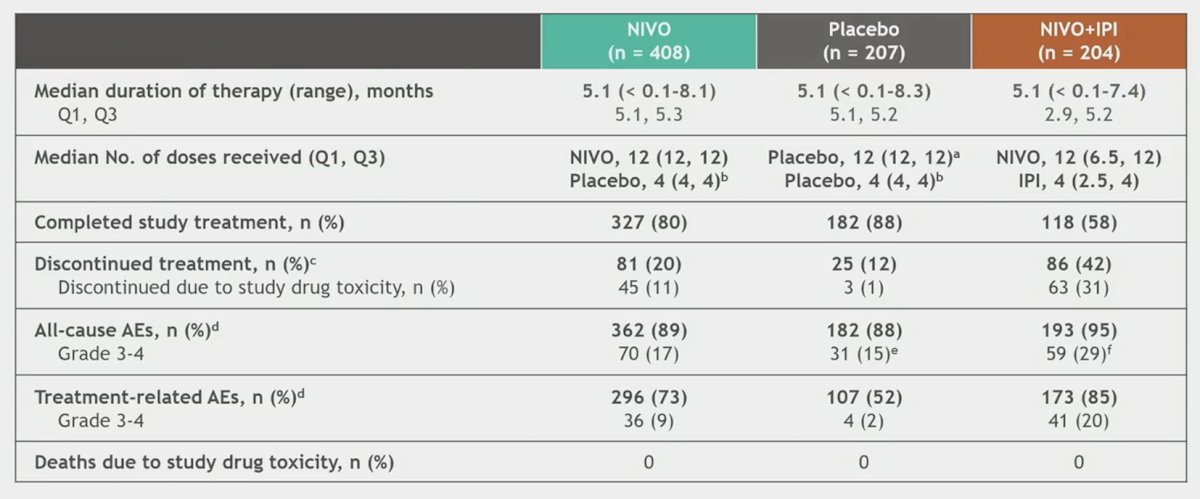
Health related quality of life was an exploratory endpoint, and the mean changes in baseline scores in the FKSI-19 (total and DRS) or EQ-5D-3L (utility index and VAS) were similar in both treatment arms and did not reach the thresholds considered meaningful based on the literature: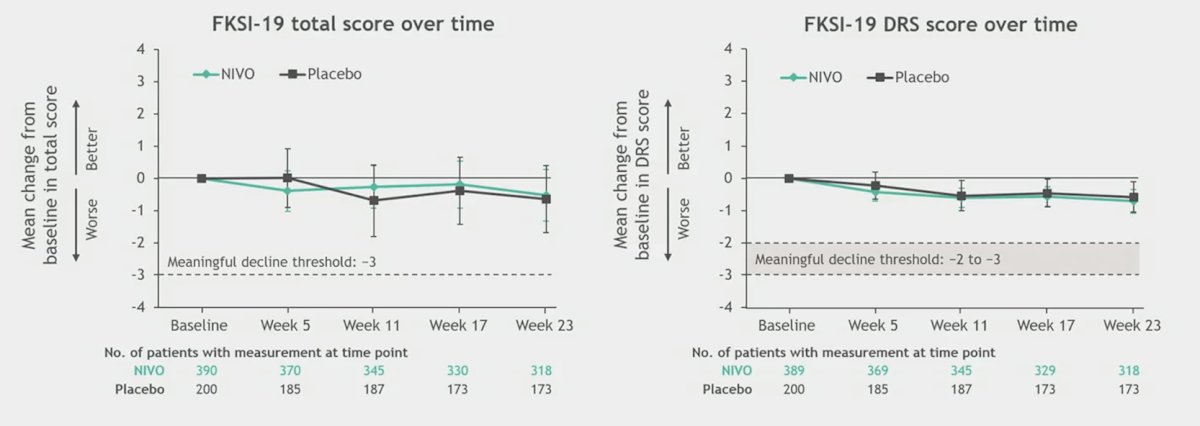
Dr. Motzer concluded his presentation discussing results from Part B of the Phase 3 CheckMate 914 trial with the following take-home points:
- CheckMate 914 Part B was designed to assess nivolumab monotherapy versus placebo in the adjuvant setting and with intent to examine contribution of components for Part A
- The primary endpoint of disease free survival for nivolumab monotherapy versus placebo was not met
- Safety of nivolumab monotherapy in this population was consistent with the profile in advanced RCC
- Subgroup analyses from CheckMate 914 Part A and Part B suggest that tumor-specific characteristics (ie. PD-L1 expression, sarcomatoid features) may influence outcomes of adjuvant nivolumab + ipilimumab and nivolumab monotherapy treatment
Presented by: Robert J. Motzer, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:


