(UroToday.com) The 2024 GU ASCO annual meeting featured a renal cell carcinoma session and a presentation by Dr. Mehul Gupta discussing treatment-free survival after first-line therapies for metastatic RCC. Novel agents targeting cancer cell growth and immune evasion have significantly improved survival for patients with metastatic RCC. However, patients treated with immune checkpoint blockade may experience disease control without need for ongoing systemic therapy, a period not well described by conventional time-to-event endpoints. An understanding of this is crucial to accurately counsel patients and aid in treatment decision making given the association between time spent free of systemic therapy and quality of life. Treatment-free survival represents a novel endpoint to quantify this period.
Dr. Gupta and colleagues identified patients with metastatic RCC from the IMDC dataset initiating first-line systemic therapy with VEGFR monotherapy (sunitinib, pazopanib), combination immune checkpoint blockade-VEGFR therapy ([axitinib or lenvatinib]-pembrolizumab, cabozantinib-nivolumab, axitinib-avelumab), or immune checkpoint blockade doublet therapy (ipilimumab-nivolumab) between February 1, 2014, and February 1, 2023. Overall survival from treatment initiation was partitioned into periods including treatment-free survival, time on first-line therapy, and survival after subsequent therapy initiation utilizing differences in restricted mean survival time over 36-months. Treatment-free survival was defined as the difference between the 36-month restricted mean survival time between:
(1) time from treatment initiation to discontinuation of first-line therapy, death, or censor at last follow-up and
(2) time from treatment initiation to subsequent therapy initiation, death, or censor at last follow-up.
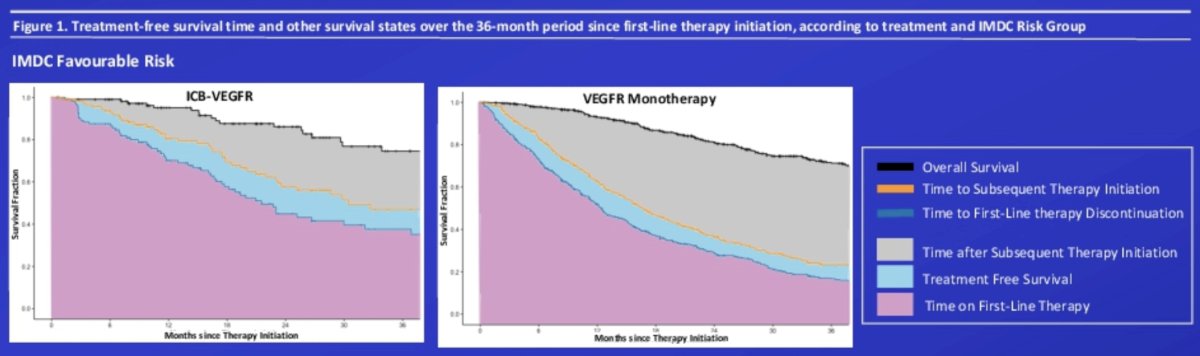
There were 3,758 patients with metastatic RCC that initiated first-line VEGFR monotherapy (n = 2,635; median age 62 years; 19.1% IMDC favorable risk), immune checkpoint blockade-VEGFR regimens (n = 354; median age 60 years; 33.3% IMDC favorable risk), or doublet immune checkpoint blockade (n = 769; median age 62 years; 0% IMDC favorable risk). Patients with favorable IMDC risk initiating VEGFR monotherapy and immune checkpoint blockade-VEGFR regimens experienced a treatment-free survival duration of 8.5% (3.1 months; 95% CI 1.5 - 4.6) and 10.1% (3.7 months; 95% CI 0.2-7.2) of the 36-month period, respectively:
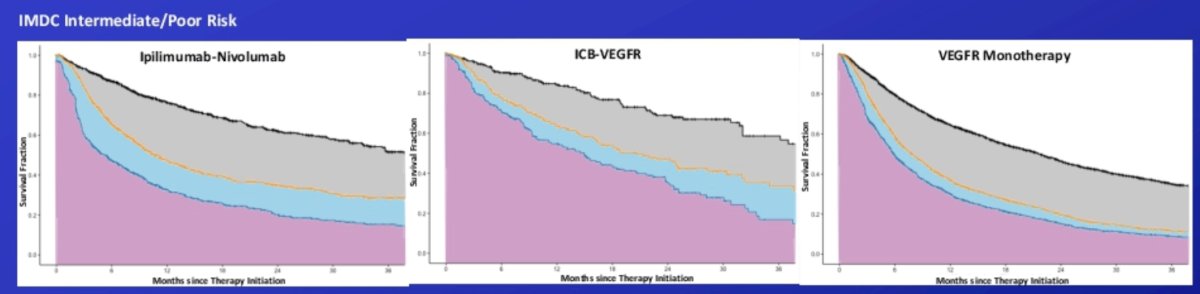
Among intermediate/poor risk patients, those treated with VEGFR monotherapy, immune checkpoint blockade-VEGFR regimens, and immune checkpoint blockade doublet therapy experienced 5.9% (2.1 months; 95% CI 1.4 - 2.8), 10.3% (4.7 months; 95% CI 1.0-6.4), and 14.6% (5.3 months 95% CI 3.8-6.8) of the 36-month period alive and treatment free, respectively:
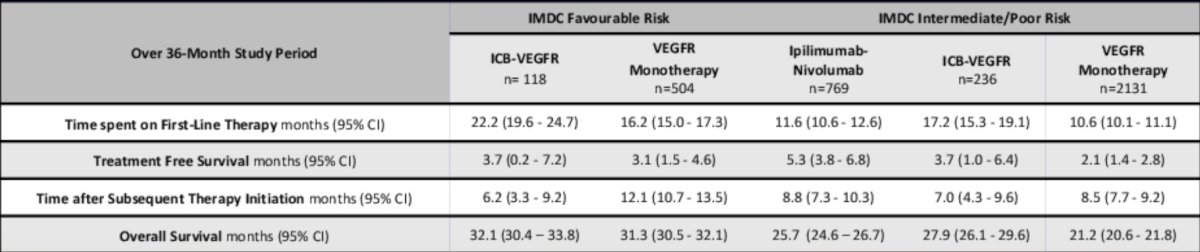
As follows is a summary table of treatment free survival states over the 36 month period since first line therapy initiation, according to treatment and IMDC risk group:
Dr. Gupta concluded his presentation discussing treatment-free survival after first-line therapies for metastatic RCC with the following take-home points:
- This study is the first observational analysis to report treatment free survival outcomes for patients with metastatic RCC receiving standard of care first line therapy
- Among IMDC favorable risk patients, despite similar overall and treatment free survival outcomes, those treated with VEGFR monotherapy spent longer on subsequent lines of treatment (17.3% vs 33.6%), while those treated with immune checkpoint blockade-VEGFR combination therapies spent longer on first line therapies (61.7% vs 44.9%)
- Intermediate/poor risk IMDC risk patients treated with immune checkpoint blockade doublet therapy experienced a treatment free survival period more than twice as long as those treated with VEGF inhibitor monotherapy over the 36 month period since therapy initiation
Presented by: Mehul Gupta, MD, Arnie Charbonneau Cancer Research Institute, University of Calgary, Calgary, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


