(UroToday.com) The 2024 GU ASCO annual meeting featured a renal cell carcinoma session and a presentation by Dr. Michael Hofman discussing the first-in-human safety, imaging, and dosimetry of [68Ga]Ga-DPI-4452, a novel carbonic anhydrase IX-targeting peptide, in patients with clear cell renal cell carcinoma (ccRCC).
In tumors, hypoxic conditions or mutation of the Von Hippel-Lindau tumor suppressor gene can induce the expression of the cell surface glycoprotein carbonic anhydrase IX. As such, carbonic anhydrase IX is overexpressed in clear cell RCC and is associated with aggressive tumor behavior, treatment resistance, and overall poor outcomes.
DPI-4452 is a first-in-class, cyclic peptide that binds with high affinity to carbonic anhydrase IX. Radiolabeling DPI-4452 with gallium-68 ([68Ga]Ga-DPI-4452) or lutetium-177 ([177Lu]Lu-DPI-4452) is an innovative, theranostic approach for identifying and treating patients with carbonic anhydrase IX-expressing tumors. Compared with existing antibody approaches, a radiolabeled peptide may confer better characteristics for both PET-CT imaging and therapy. This first-in-human study is evaluating the theranostic potential of [68Ga]Ga-DPI-4452 and [177Lu]Lu-DPI-4452 in patients with unresectable metastatic clear cell RCC, colorectal cancer or pancreatic ductal adenocarcinoma tumors. At GU ASCO 2024, Dr. Hofman reported safety, tolerability, pharmacokinetics, dosimetry, and imaging characteristics of [68Ga]Ga-DPI-4452 from the completed clear cell RCC imaging cohort.
The DPI-4452 peptide contains a DOTA cage and is radiolabeled with [68Ga]Ga. Following intravenous injection of [68Ga]Ga-DPI-4452, patients underwent serial PET-CT imaging, urine, and blood sampling to assess imaging characteristics, biodistribution, and dosimetry of [68Ga]Ga-DPI-4452. Safety, assessed by incidence of treatment emergent adverse events, was evaluated in patients over a seven day post-injection period:![]()
There were three patients with metastatic clear cell RCC, all male, that were enrolled in the Part A imaging cohort of the study, with a mean activity of 189.9 +/- 13.74 MBq [68Ga]Ga-DPI-4452 administered:![mean activity of 189.9 +/- 13.74 MBq [68Ga]Ga-DPI-4452 administered](/images/com-doc-importer/145-asco-gu-2024/asco-gu-2024-first-in-human-safety-imaging-and-dosimetry-of-68ga-ga-dpi-4452-a-novel-ca-ix-targeting-peptide-in-patients-with-clear-cell-rcc/image-1.jpg)
No clinically significant toxicities were observed. PET-CT images showed rapid and sustained tumor uptake over 4 hours, as well as rapid renal elimination. At 1 hour, the maximum tumor standardized uptake value (SUVmax) across 36 lesions ranged from 6.8 to 211.6, with a mean of 64.6 (SD 54.8). Seventeen of these lesions (found in lymph nodes, lung, pancreas, parotid gland, and other sites) were not detectable on prior contrast-enhanced CT: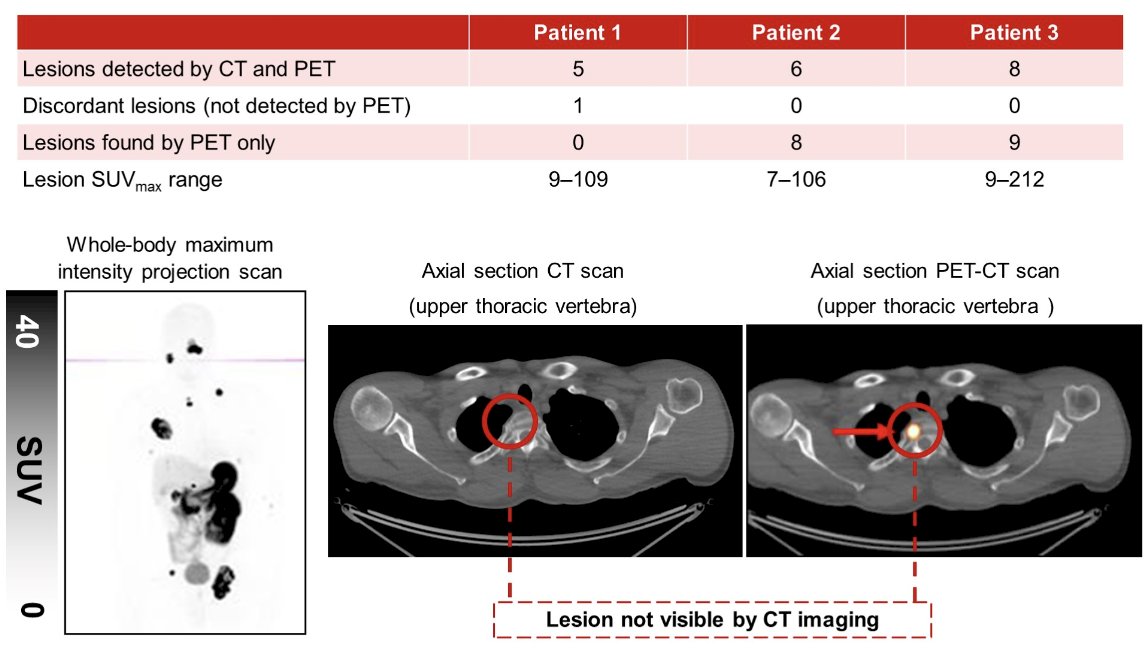
Over 80% of total administered radioactivity cleared from the bloodstream within 1 hour. Between early and late time intervals, the average percentage injected dose in urine declined from 13.3 (SD 4.5) to 6.1 (SD 3.6):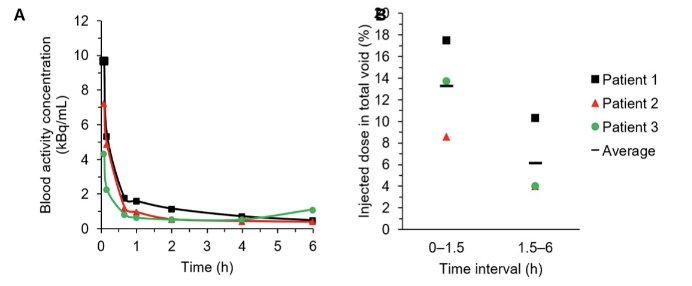
OLINDA dosimetry estimates revealed that the organs receiving the highest absorbed doses (mGy/MBq) were stomach wall (mean 0.33, SD 0.10), small intestine wall (mean 0.33, SD 0.08), and gallbladder wall (mean 0.21, SD 0.12), with a mean whole body effective dose of mean 0.06 (SD 0.02) mSv/MBq. Absorbed doses in the kidney, liver and bone marrow were low. Two grade 1 treatment emergent adverse events were reported in two patients (increased blood creatinine phosphokinase and headache), though neither were causally related to [68Ga]Ga-DPI-4452 administration.
Dr. Hofman concluded his presentation discussing the first-in-human safety, imaging and dosimetry of [68Ga]Ga-DPI-4452 in patients with clear cell RCC with the following take-home points:
- [68Ga]Ga-DPI-4452 provides exceptional images in patients with clear cell RCC without clinically significant toxicity
- Very high SUVs and tumor-to-background ratios suggest potential for use in both diagnostics and patient selection for therapy
- The tumor retention and rapid elimination support potential of [177Lu]Lu-DPI-4452 radioligand therapy
- These first-in-human findings with radiolabeled DPI-4452 are encouraging for the subsequent evaluation of treatment with [177Lu]Lu-DPI-4452
Presented by: Michael S. Hofman, MD, Peter MacCallum Cancer Centre, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.


