(UroToday.com) The 2024 GU ASCO annual meeting featured a renal cell carcinoma session and a presentation by Dr. Viktor Grünwald discussing subgroup analyses of efficacy outcomes by baseline tumor size in the phase 3, open-label CLEAR trial. In the primary analysis of this trial, lenvatinib + pembrolizumab showed statistically significant and clinically meaningful improvements in progression-free survival, overall survival, and objective response rate compared with sunitinib in patients with advanced RCC.1 Outcomes were further corroborated by results of the final prespecified analysis of CLEAR presented by Dr. Motzer at ASCO 2023:
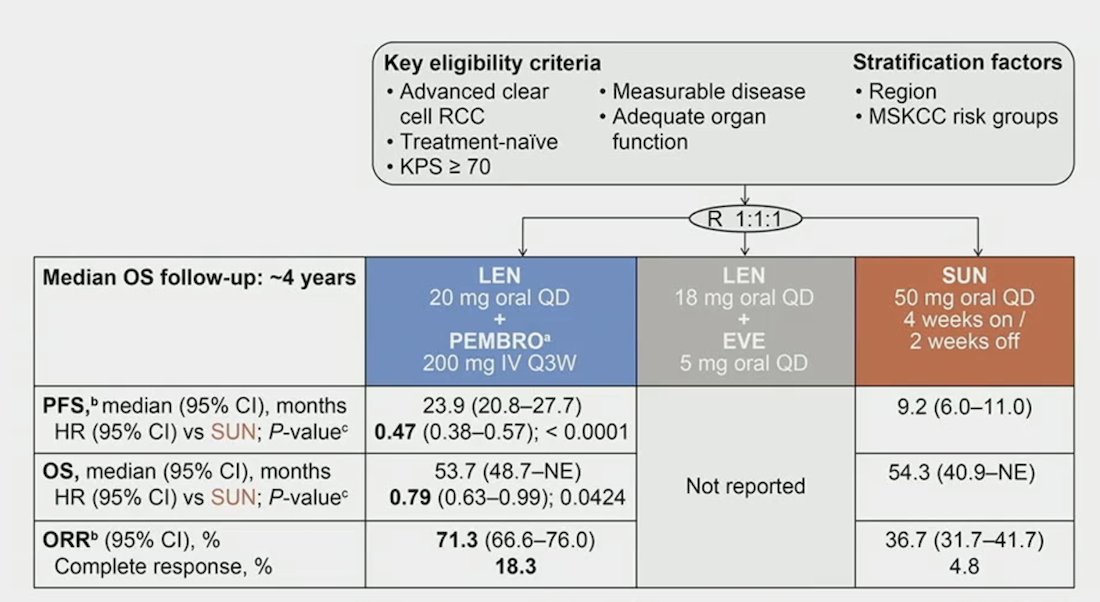
At the 2024 GU ASCO annual meeting, Dr. Grünwald reported efficacy outcomes per baseline tumor size in the lenvatinib + pembrolizumab arm of CLEAR.
There were 1,069 treatment-naïve patients with advanced RCC and a clear-cell component were randomized (1:1:1) to: lenvatinib 20 mg PO QD + pembrolizumab 200 mg IV Q3W vs lenvatinib 18 mg + everolimus 5 mg PO QD vs sunitinib 50 mg PO QD (4 weeks on/2 weeks off). Stratification factors included geographic region and MSKCC prognostic risk group, and IMDC risk groups (derived programmatically) are presented in this analysis. Efficacy outcomes (data cutoff date: July 31, 2022) were evaluated by quartiles of baseline sums of diameters of target lesions. Tumor assessments were performed by independent imaging review per RECIST v1.1.
Patients were grouped into 4 categories by baseline sums of diameters of target lesions:
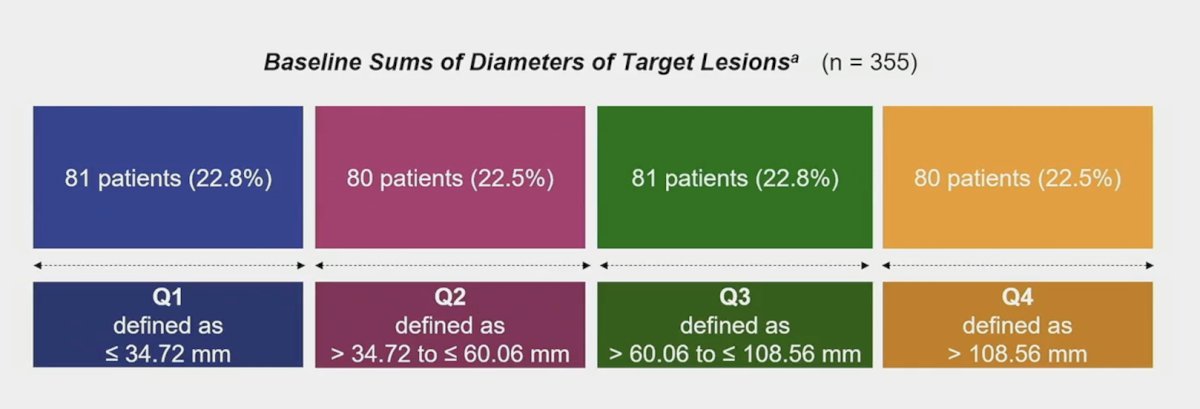
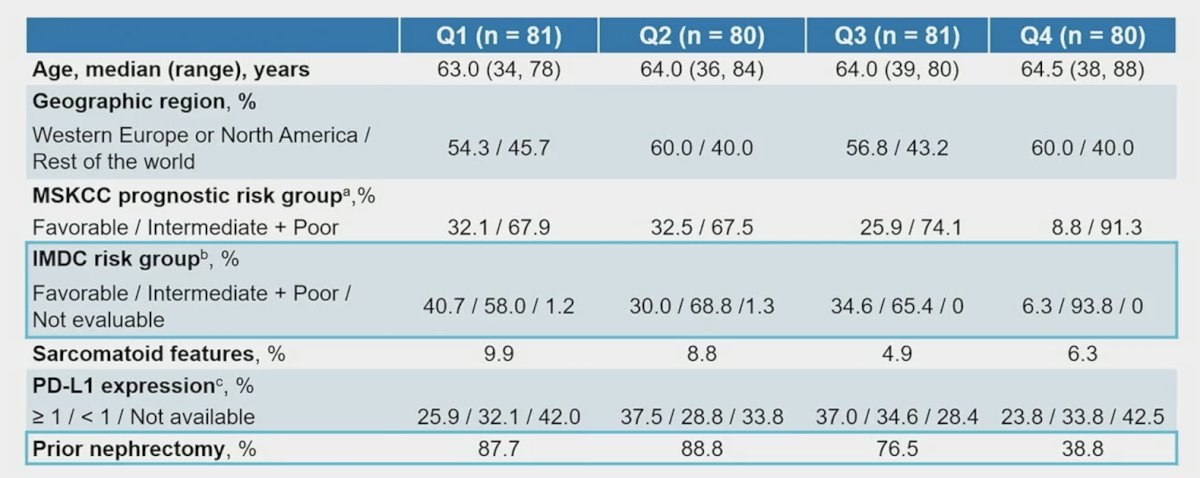
For patients with Q1 baseline tumor sizes, 40.7% and 58.0% were in the favorable and intermediate + poor IMDC risk subgroups, respectively. For patients with 43 baseline tumor sizes, 6.3% and 93.8% were in the favorable and intermediate + poor IMDC risk groups, respectively. The full baseline characteristics by Q1 through Q4 are as follows:
Overall survival by tumor size showed that the 3 year survival rates for Q1 was 73.0%, 69.3% for Q2, 63.4% for Q3, and 55.6% for Q4:

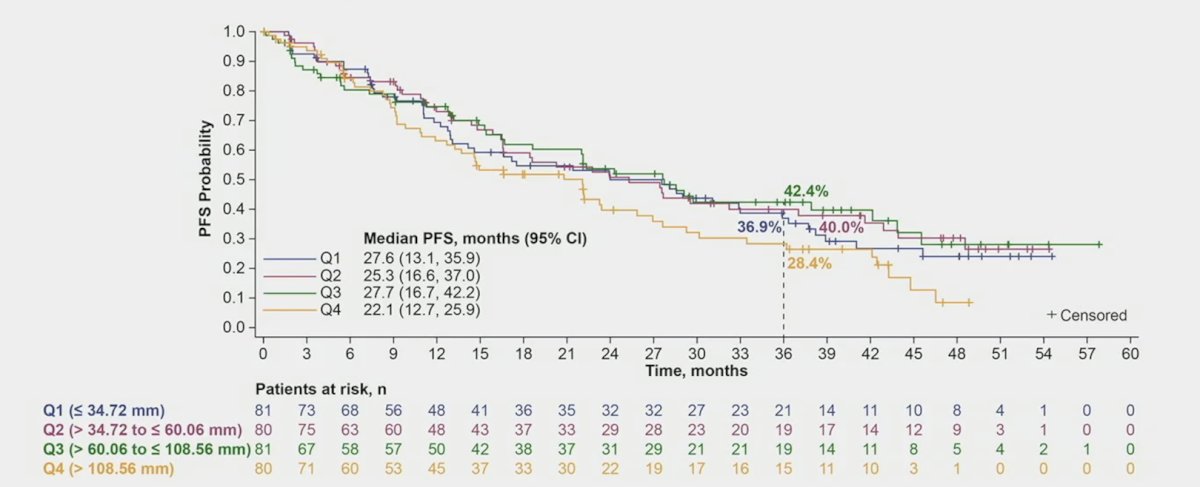
Progression free survival by tumor size showed that the 3 year rates for Q1 was 36.9%, 40.0% for Q2, 42.4% for Q3, and 28.4% for Q4:
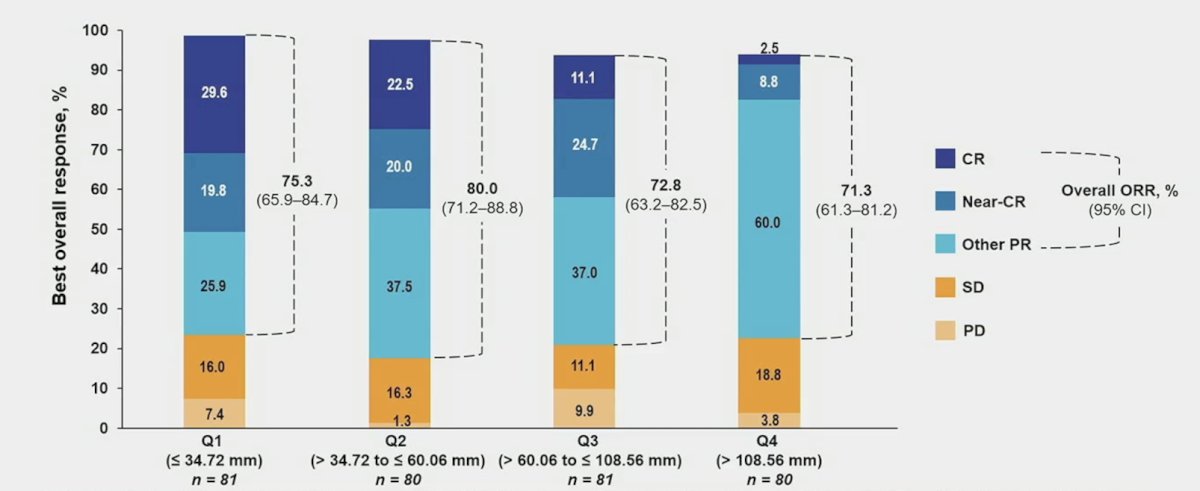
The best overall response by tumor size showed an objective response rate of 75.3% for Q1, 80.0% for Q2, 72.8% for Q3, and 71.3% for Q4:
Dr. Grünwald concluded his presentation discussing subgroup analyses of efficacy outcomes by baseline tumor size in the phase 3, open-label CLEAR trial with the following take-home points:
- With extended follow-up (median ~4 years) of the CLEAR study, progression free survival, overall survival, and objective response rate benefits with lenvatinib + pembrolizumab were observed across patients with advanced RCC irrespective of their baseline tumor sizes
- Taken together, these results support the use of lenvatinib + pembrolizumab for the first line treatment of advanced RCC across patients with both low and high baseline tumor size
Presented by: Viktor Grünwald, MD, University Hospital Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


