(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a trials in progress renal cell, adrenal, penile, urethral, and testicular cancers poster session. Dr. Raquibul Hannan presented the EA8211-SOAR, a phase 3 randomized trial of stereotactic ablative radiotherapy (SABR) for oligometastatic advanced renal cell carcinoma (RCC).
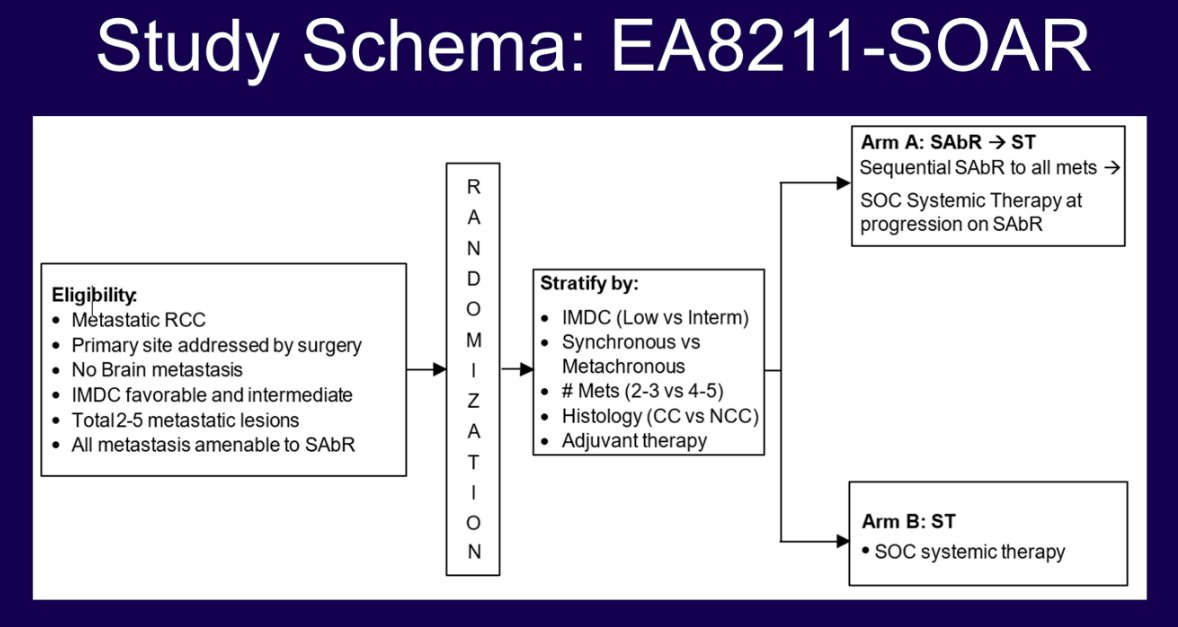
The optimal strategy for managing oligometastatic renal cell carcinoma (RCC) remains unclear. While systemic therapy with immune checkpoint inhibitors is currently considered standard of care, SABR is a promising alternative based on retrospective and limited prospective data. It has been hypothesized that SBRT may spare patients systemic therapy and associated toxicities, but concerns remain regarding the risk of occult micrometastatic disease progression. The prospective ECOG-ACRIN phase 3 randomized trial aims to compare SABR to systemic therapy for patients with metachronous (i.e., primary site pre-treated) oligometastatic RCC.
In this open label, noninferiority, randomized phase 3 trial, patients meeting the eligibility criteria will undergo stratified randomization to either:
- Arm A: Sequential SABR to all metastatic sites, with standard of care systemic therapy reserved for patients with progressive disease
- Arm B: Standard of care systemic therapy
Eligibility criteria are as follows:
- Two to five sites of measurable metastases
- Primary site addressed by local therapy (surgery or radiation)
- IMDC favorable and intermediate risk disease
- All metastases amenable to SBRT
Key exclusion criteria are as follows:
- Brain metastases
- Previous systemic therapy for metastatic disease (adjuvant therapy allowed)
- Sarcomatoid histology
- IMDC poor risk disease
The co-primary endpoints are overall survival and Grade 3 toxicity. Key secondary endpoints include:
- Quality of life (NFKSI-19 and EQ-5D-5L)
- Progression-free survival
- Cost-effectiveness
- ctDNA correlative analysis
The target sample size of 472 patients will allow for 85% power test the non-inferiority of overall survival in the SABR arm, assuming a null hazard ratio of 1.24 and an alternative of 0.85.
Presented by: Raquibul Hannan, MD, PhD, Professor, Chief of Genitourinary Radiation Oncology, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.


