(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a renal and rare tumors oral abstract session. Dr. Saby George presented the late-breaking pharmacokinetics, efficacy, and safety results from CheckMate 67T comparing subcutaneous and intravenous nivolumab in patients with previously treated advanced or metastatic clear cell renal cell carcinoma (RCC).
Evolving treatment paradigms require administration options that decrease treatment burden for patients and improve the efficiency of healthcare systems. The subcutaneous delivery of antibodies for various cancer indications have proved to be effective and well-tolerated. Furthermore, subcutaneous administration is typically preferred to intravenous infusion by patients and has improved healthcare resource utilization by decreasing preparation and chair occupancy time, as well as reducing administrative burden.
Subcutaneous nivolumab (NIVO SC) is co-formulated with rHuPH20, which reversibly degrades hyaluronan in the extracellular matrix around the SC injection area enabling administration of large volumes. Various doses of NIVO SC were previously assessed in the dose-finding phase 1/2 study CheckMate 8KX.1 Population pharmacokinetic analysis of patients treated in CheckMate 8KX and historical NIVO IV data were used to determine a flat dose of 1,200 mg NIVO SC for further investigation.2 This dose provides NIVO exposure that is within the known safe and efficacious therapeutic treatment window of 1-10 mg/kg NIVO IV every 2 weeks and accounts for a range of bodyweights, while mitigating potentially low bioavailability when administered to a broad patient population.
CheckMate 67T (NCT04810078) is a multicenter, randomized, open-label, phase 3 study that evaluated the pharmacokinetics and objective response rate (ORR) non-inferiority of NIVO SC versus IV in patients with locally advanced or metastatic ccRCC treated in the 2nd or 3rd line settings.
This non-inferiority randomized trial included patients with advanced or metastatic clear cell RCC that progressed during or after receiving 1-2 prior systemic regimens and had not received prior immune-oncology therapy. Patients underwent 1:1 randomization to either NIVO SC 1,200 mg + rHuPH20 every 4 weeks (n=248) or NIVO 3mg/kg every 2 weeks (n=247). Treatment was continued until disease progression, unacceptable toxicity, consent withdrawal, completion of two years of treatment, or death.
The co-primary pharmacokinetic endpoints for non-inferiority testing were:
- Average concentration at 28 days (Cavgd28)
- Minimum serum concentration at steady state (Cminss)
The key powered secondary endpoint for non-inferiority testing was ORR by blinded independent central review (BICR). Other secondary endpoints included:
- Other efficacy, safety, and pharmacokinetic measures
- Incidence of anti-NIVO antibodies and neutralizing antibodies

The definitions of the pharmacokinetic endpoints and the statistical methods/design for this non-inferiority comparison are summarized below:

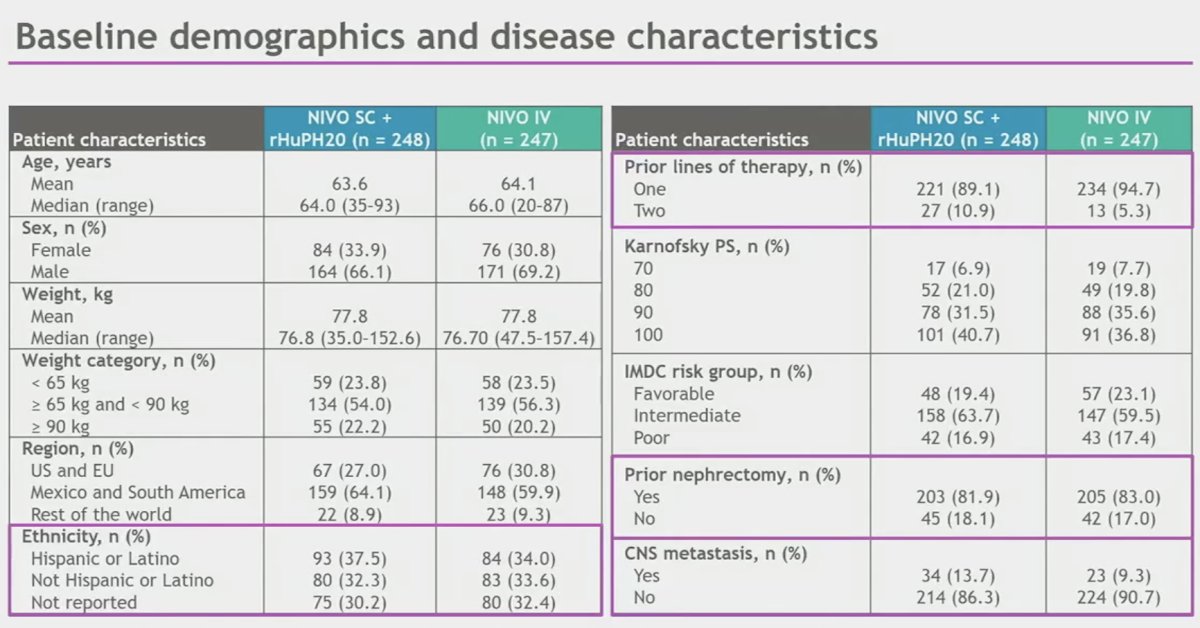
The baseline patient characteristics are summarized below. This was a diverse cohort of patients with over a third of patients identifying as Hispanic or Latino. The vast majority (~90%) had received one prior line of therapy. Approximately 82% had undergone a prior nephrectomy. Central nervous system metastases were present in 11.5% of patients.
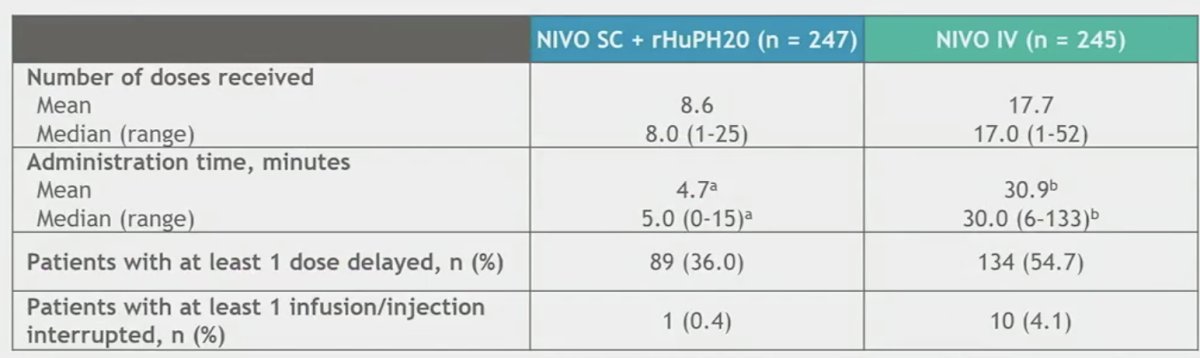
The average administration time with NIVO SC was ≤5 minutes. Most patients received all doses of the study medications without an infusion/injection interruption or dose delay.
The most common reason for dose delays of treatment was adverse events.
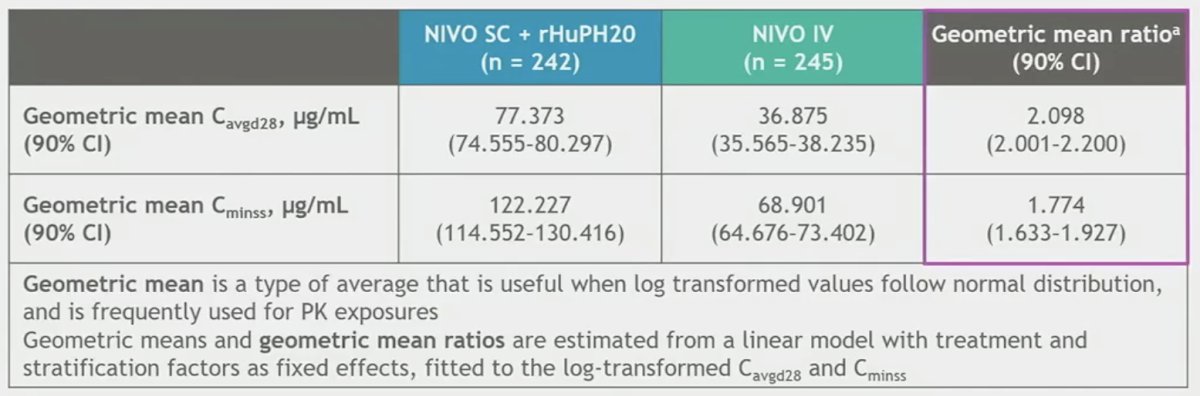
Non-inferiority for the co-primary pharmacokinetic endpoints was met.
Similarly, non-inferiority for the key powered secondary endpoint ORR by BICR was met, with an ORR of 60% in the subcutaneous arm versus 45% in the intravenous arm (RR: 1.33, 95% CI: 0.94 – 1.87).

With regards to the other secondary efficacy endpoints, the disease control rate was similar for both arms (~63%) and the median time-to-response was 3.7 for both.
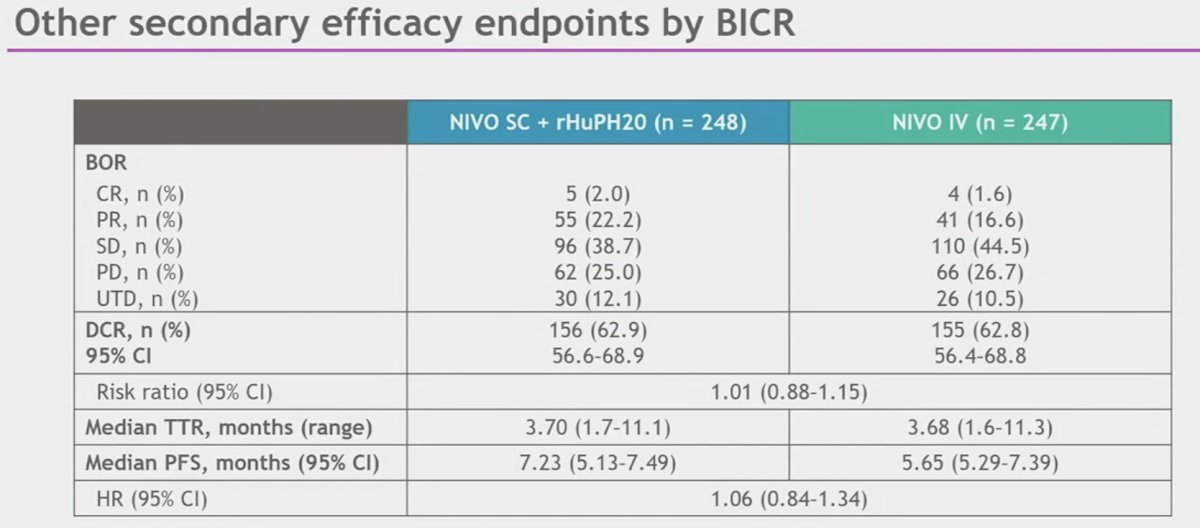
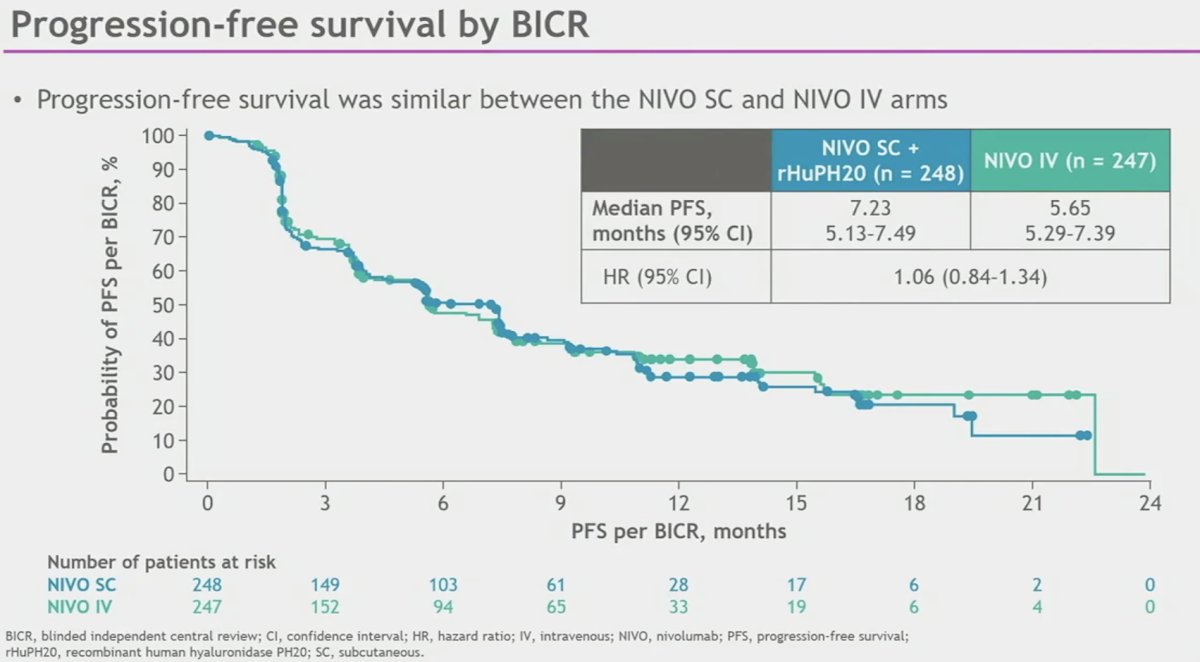
While the median PFS was longer with NIVO SC (7.2 versus 5.7 months), this difference did not meet statistical significance (HR: 1.06, 95% CI: 0.84 – 1.34).
Data from further immunogenicity assessments did not identify apparent clinically meaningful impact of development of anti-NIVO antibodies on pharmacokinetics, efficacy, or safety

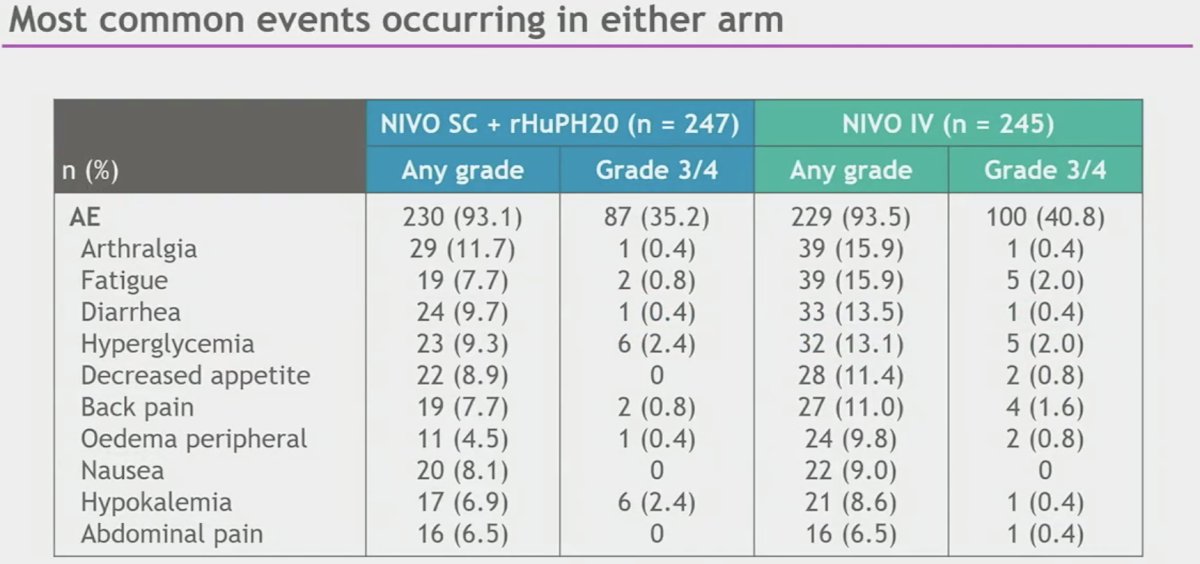
Safety was consistent between NIVO IV and NIVO SC. The rates of adverse events (AEs), treatment-related AEs, serious AEs, treatment-related AEs, AEs leading to discontinuation, and treatment-related AEs leading to discontinuation for SC arm were similar or lower than the IV arm.
Study drug toxicity led to three deaths in the NIVO SC arm and one death in the NIVO IV arm. Local site reactions in the NIVO SC arm were low grade, transient (mean duration of 3 days), and most resolved without treatment. There were no anaphylactic reactions observed in either arm.

Dr. George concluded that:
- CheckMate 67T met its co-primary endpoints and key powered secondary endpoint; NIVO SC demonstrated noninferiority of exposures (Cavgd28 and Cminss) and efficacy (ORR by BICR) versus NIVO IV
- PFS was similar between the NIVO SC and NIVO IV arms
- NIVO SC was also consistent with NIVO IV across additional secondary efficacy endpoints, including disease control rate and time to response
- The safety profile of NIVO SC was consistent with the safety profile of NIVO IV with no new safety concerns or signals identified
- Local site reactions were low grade, transient, and most resolved without treatment
- No increase in hypersensitivity reactions or risk of anaphylaxis was observed
- NIVO-related immunogenicity results were as expected for SC route of administration and further assessments did not identify apparent clinically meaningful impact
- Average administration time with NIVO SC was ≤ 5 minutes
- These data indicate that NIVO SC provides clinical equipoise to standard IV dosing, supporting the use of NIVO SC as a new option to reduce patients' treatment burden and improve healthcare efficiency
Presented by: Saby George, MD, FACP, Associate Professor, Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:1. Lonardi S, et al. Poster presentation at the SITC Annual Meeting; November 1-5, 2023; San Diego, CA.
2. Zhao Y, et al. Model-Based Dose Selection of Subcutaneous Nivolumab in Patients with Advanced Solid Tumors. Clin Pharmacol Ther. 2024.
Related Content: Read the Discussion: ASCO GU 2024: Insights from CheckMate 67T and Patient-Reported Outcomes in LITESPARK-005 for Advanced RCC
CheckMate-67T: Subcutaneous Nivolumab Shows Comparable Results to IV, Reduces Treatment Burden in RCC - Saby George


