(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a renal and rare tumors oral abstract session. Dr. Thomas Powles presented the patient-reported outcomes from the phase 3 LITESPARK-005 study of belzutifan versus everolimus in patients with previously treated advanced renal cell carcinoma (RCC).
Belzutifan is a first-in-class Hypoxia-Inducible Factor-2α (HIF-2α) inhibitor and has demonstrated anti-tumor activity in previous treated advanced clear cell RCC.1 The randomized, phase 3 LITESPARK-005 study showed significant improvement in PFS and ORR with belzutifan versus everolimus in patients with advanced clear cell RCC after prior immune checkpoint and anti-angiogenic therapies, prolonging the primary endpoint of PFS (HR: 0.75, 95% CI: 0.63 – 0.90, p<0.001) and improving ORR (key secondary end point), with an estimated difference of 18.4% (p<0.001).2
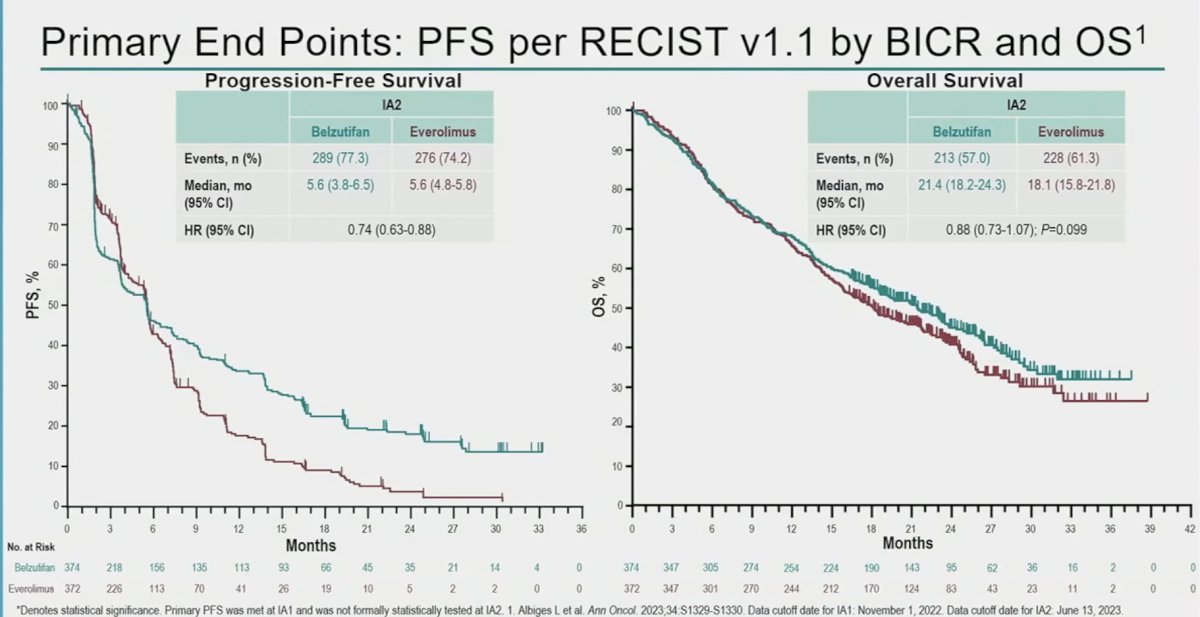
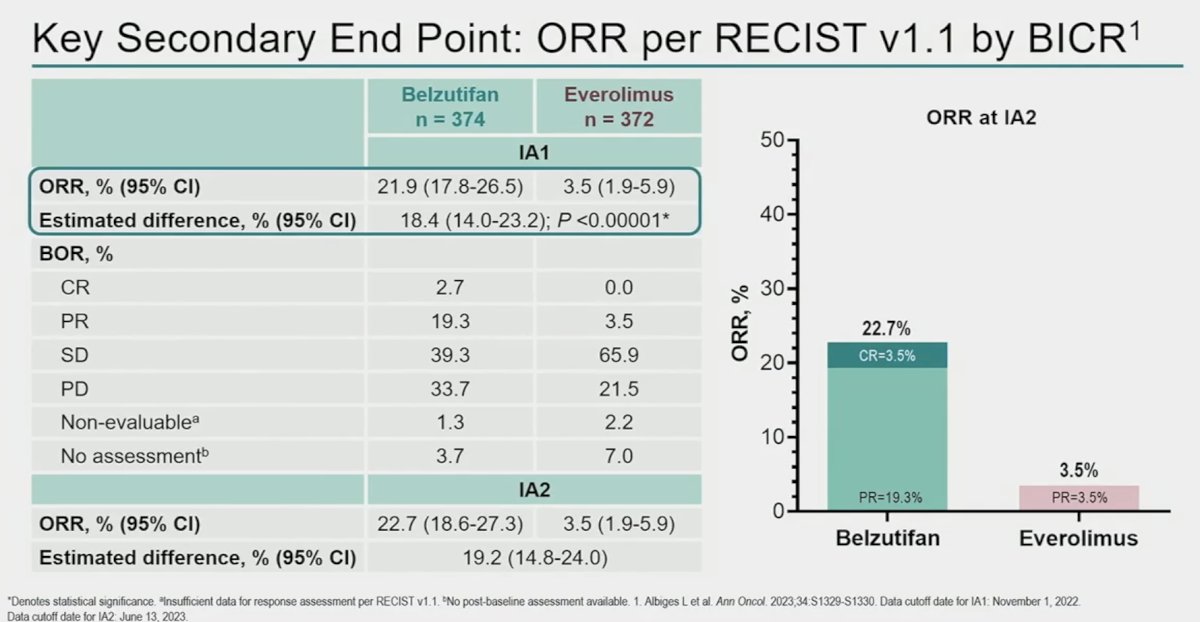
Based on the results of LITESPARK-005 presented at ESMO 2023, belzutifan was approved by the FDA for the treatment of adult patients with advanced RCC following a PD-1/L1 inhibitor and a VEGF-TKI. In this analysis, Dr. Powles presented the patient-reported outcomes (PROs) for belzutifan versus everolimus from LITESPARK-005.
The study design for LITESPARK-005 is summarized below:
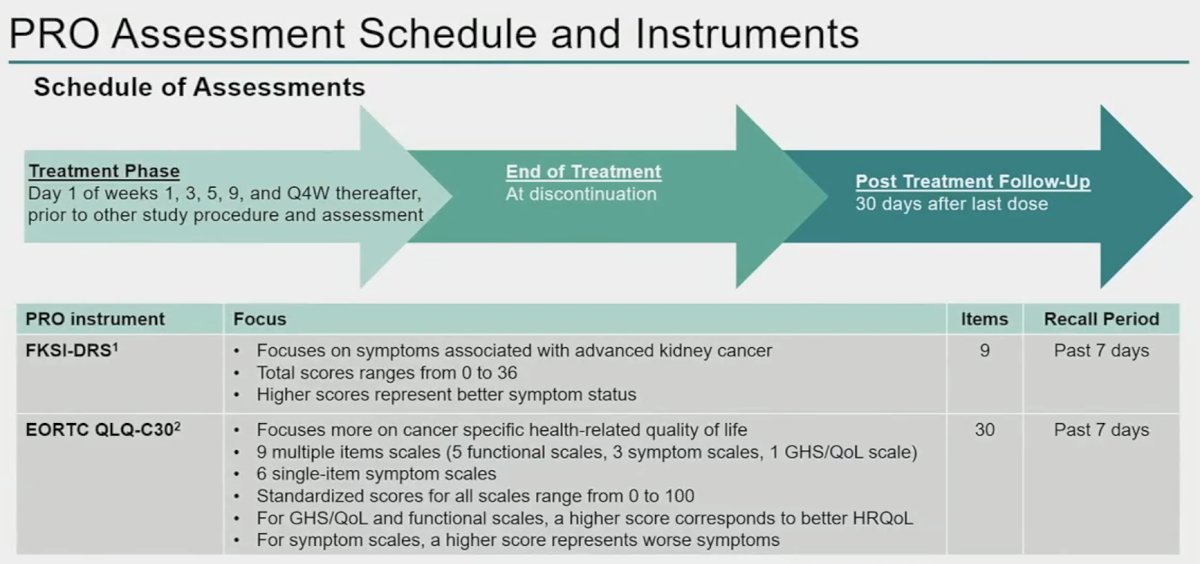
PROs were assessed during the treatment phase on day 1 of weeks 1, 3, 5, and 9, and every 4 weeks thereafter, at the end of treatment, and 30 days post-treatment. The validated Functional Assessment of Cancer Therapy Kidney Symptom Index (FKSI-DRS) and European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire 30 (EORTC QLQ-C30) were used for assessment of PROs.
Secondary end points included:
- Time to confirmed deterioration (TTD), defined as time from baseline to first onset of score worsening by ≥3 points for FKSI-DRS or ≥10 points for EORTC QLQ-C30 with confirmation at the subsequent visit
- Mean score change from baseline to week 17 in HRQoL as assessed by the FKSI-DRS and EORTC QLQ-C30 GHS/QoL, and QLQ-C30 Physical and Role Functioning subscales
Exploratory analyses included:
- Mean change from baseline over time for FKSI-DRS and EORTC QLQ-C30 GHS/QoL, up to week 137
- Mean change from baseline to week 17 in all other EORTC QLQ-C30 subscales and symptom scores
- Proportion of participants with overall improvement/stability/deterioration for FKSI-DRS and EORTC QLQ-C30
The PRO analysis population included all randomized participants who received ≥1 dose of study treatment and completed ≥1 PRO assessment. Differences between treatment groups were statistically assessed as follows:
- Time to deterioration: HR estimation using stratified Cox regression model with Efron's method of handling ties
- Least squares mean score change from baseline: Constrained longitudinal data analysis (cLDA) model with PRO scores as the response variable, and treatment-by-time interaction and stratification factors as covariates
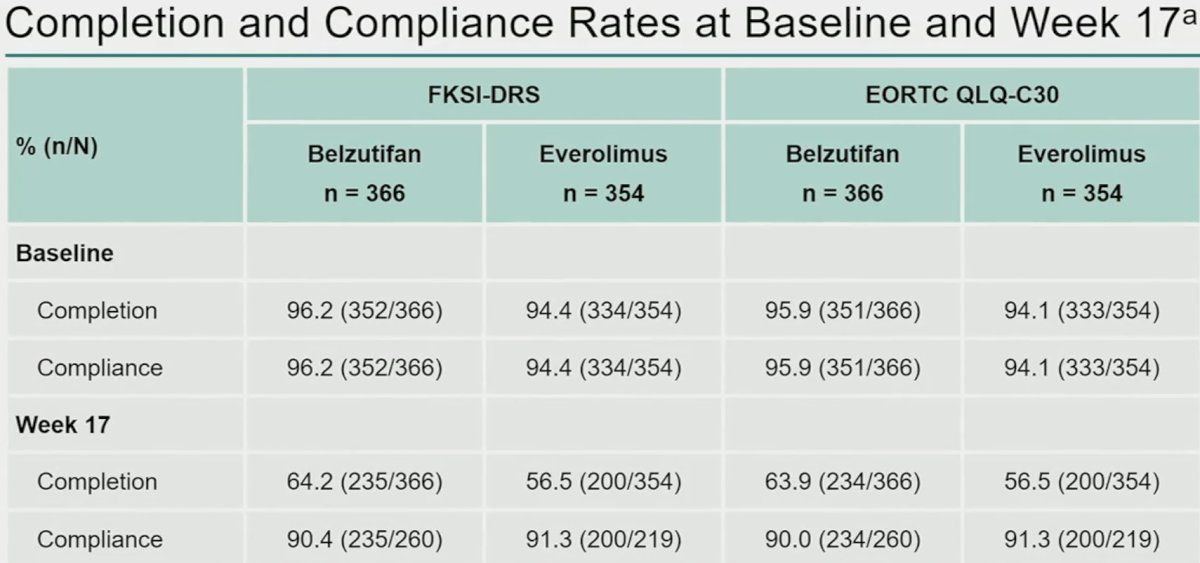
The completion and compliance rates at baseline were all >94%. By week 17, the completion rates had declined to approximately 60%, as is commonly observed in trials with longitudinal repeated assessment.
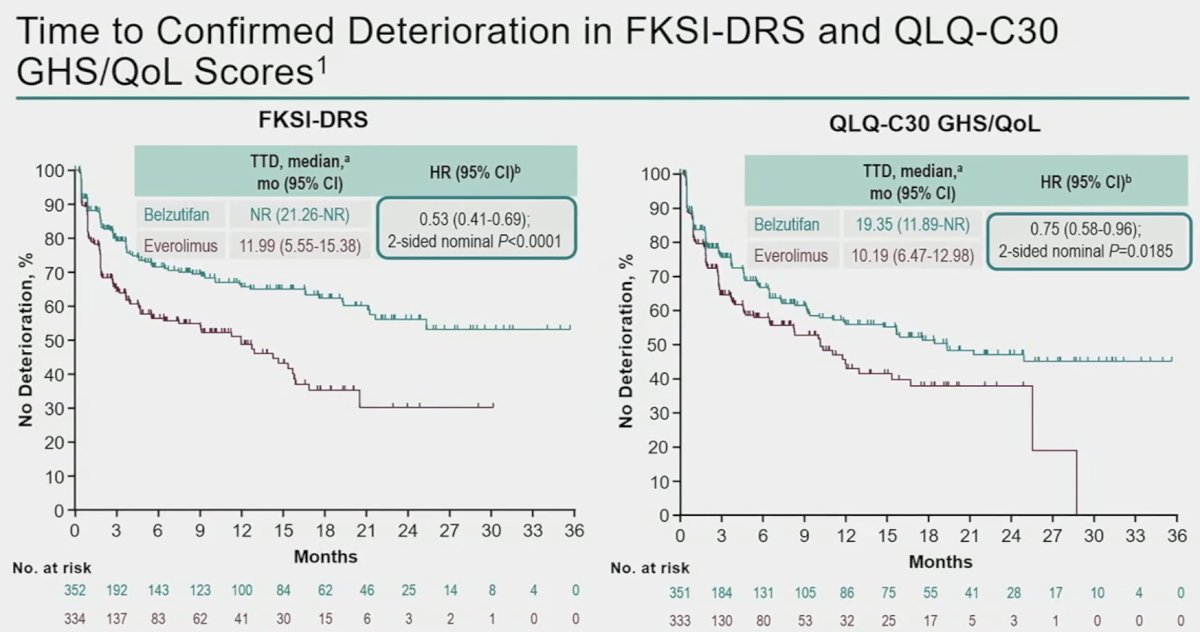
Compared to everolimus, belzutifan was associated with improved time to confirmed deterioration, as assessed by both the disease-specific (FKSI-DRS) and global (QLQ-C30 GHS/QoL) questionnaires.
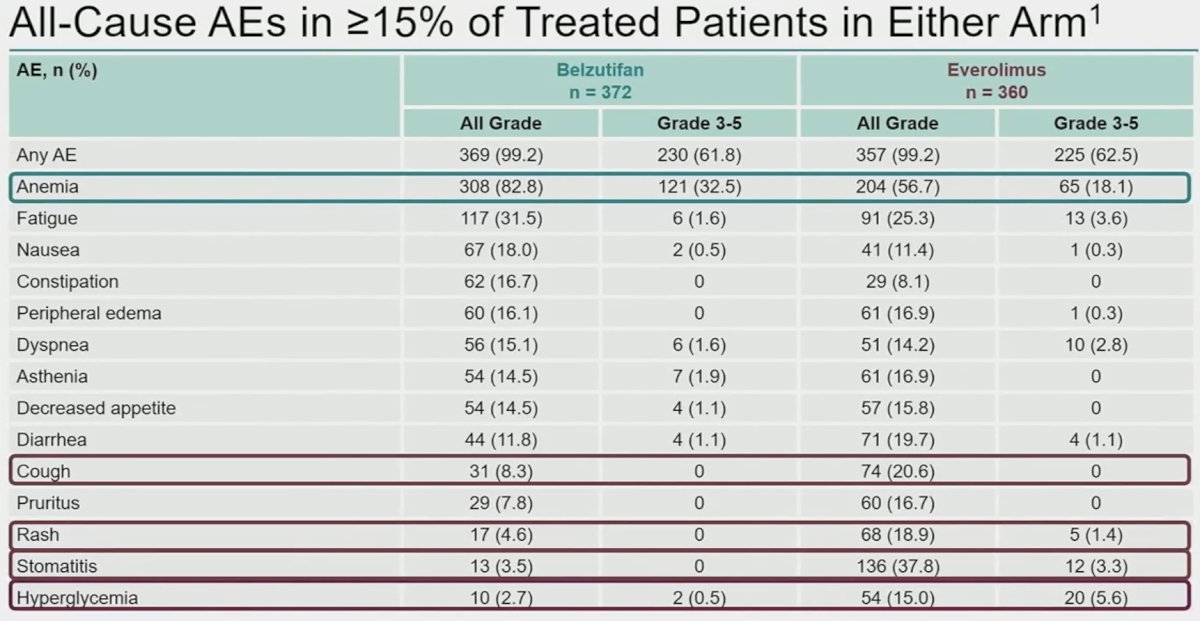
Although the rates of any grade and grades 3 – 5 adverse events were similar between both treatments, the patterns of adverse events were significantly different. Anemia was the major adverse event of interest occurring much more commonly with belzutifan (Grade 3: 33% versus 18%), whereas cough, rash, stomatitis, and hyperglycemia were more common with everolimus.
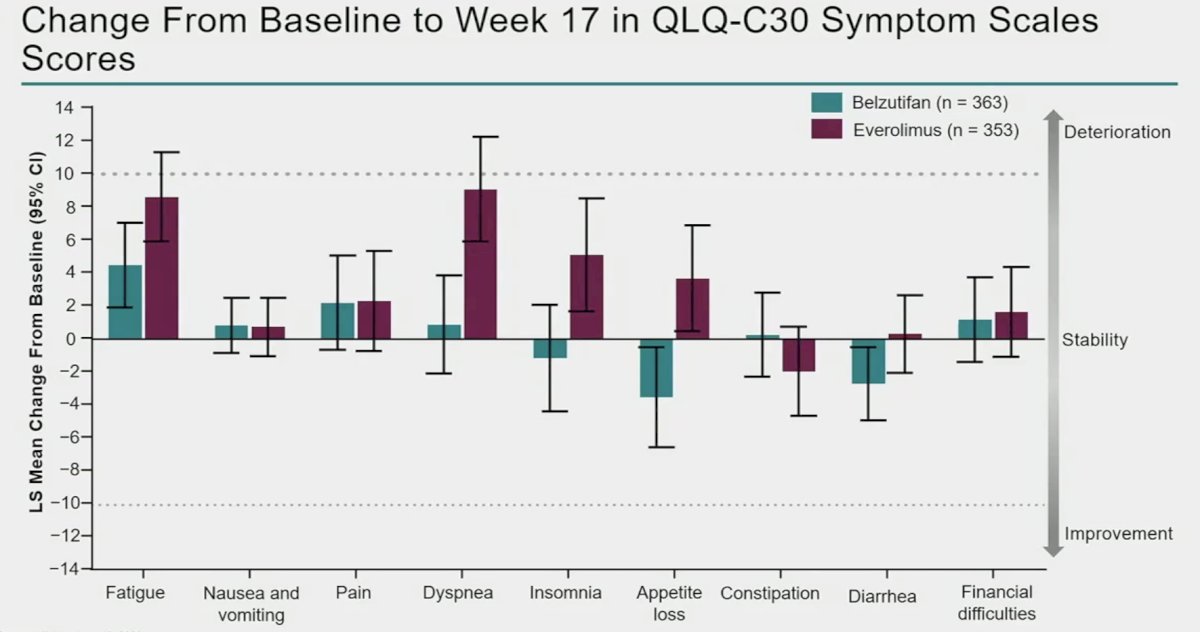
How do these adverse events correlate with global quality of life? In the graph below, the least squares mean change from baseline for each individual adverse event is summarized (upwards: deteriorated, downwards: improved). Overall, belzutifan had a less significant deterioration in global quality of life for fatigue, dyspnea, insomnia, appetite loss, and diarrhea.
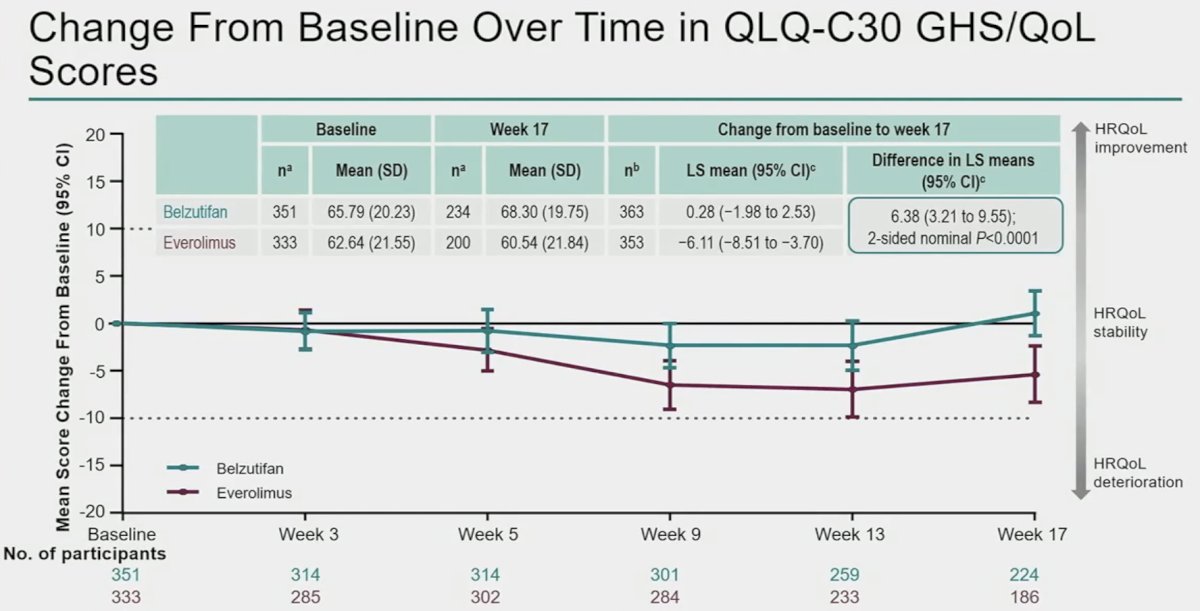
When changes from baseline were assessed over time using the disease-specific FKSI-DRS and the global QLQ-C30 GHS/QoL questionnaires, Dr. Powles noted overall symptom stability for patients on belzutifan with minimal deterioration observed.
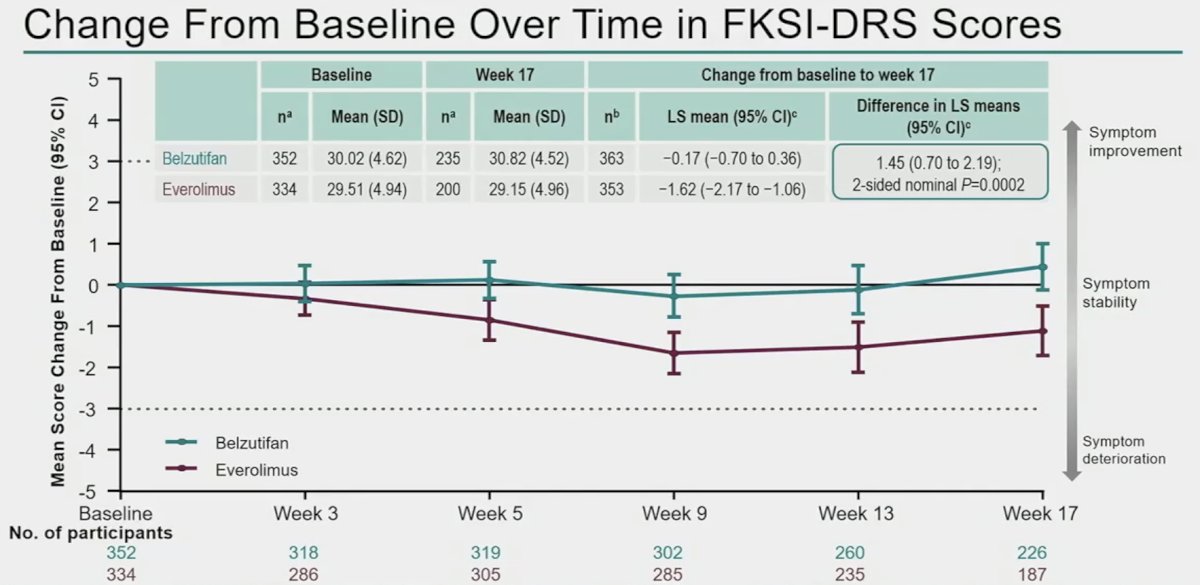
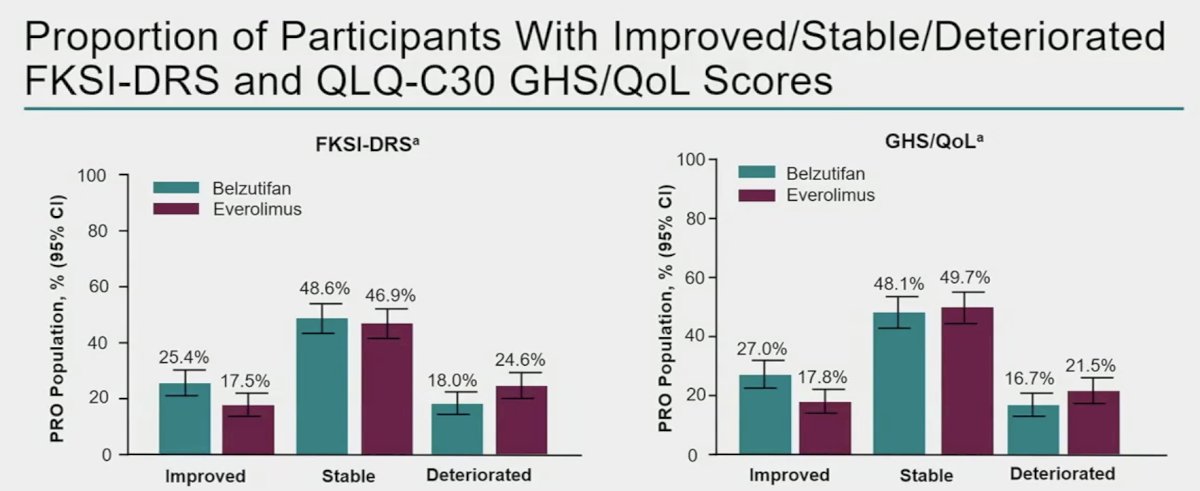
Improvements in symptom scores were more common with belzutifan (25 – 27% versus 17 – 18%).
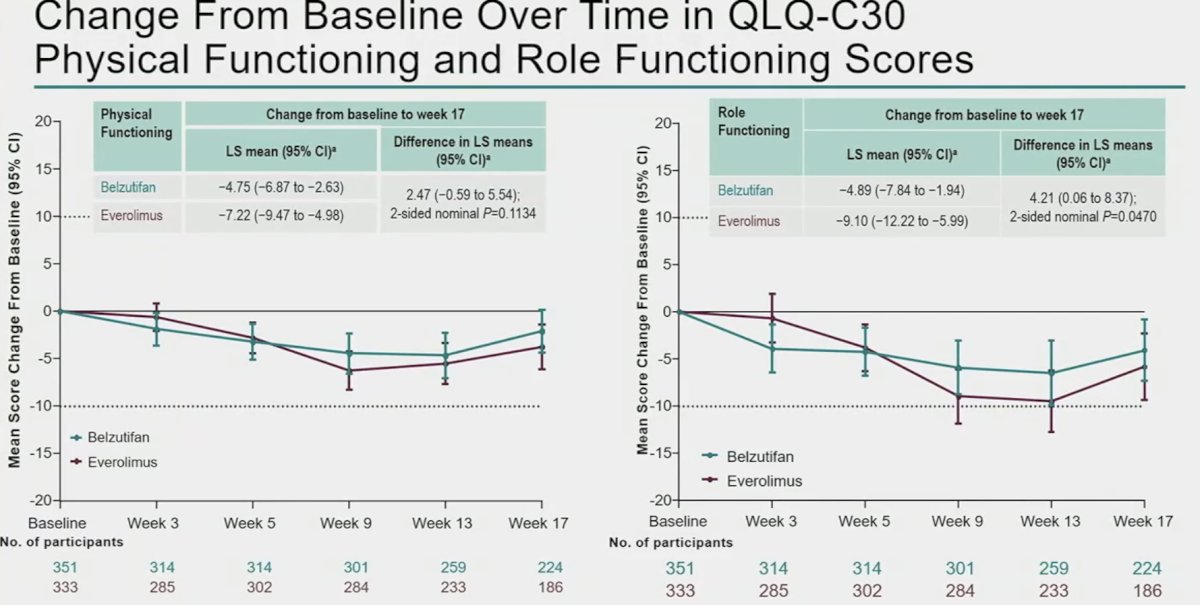
Change from baseline over time in QLQ-C30 physical functioning and role functioning scores both slightly favored belzutifan over everolimus.
Dr. Powles concluded as follows:
- Patient reported outcomes results indicated better disease-specific symptoms and quality of life with belzutifan compared with everolimus in patients with advanced clear cell RCC
- Belzutifan was associated with meaningfully longer time to deterioration in the disease-specific FKSI-DRS and global EORTC QLQ-C30 GHS/QoL scales, compared with everolimus
- Overall, least squares and empirical mean changes from baseline in FKSI-DRS and EORTC QLQ-C30 suggested greater worsening in the everolimus group versus stability in the belzutifan group
- Along with improvement in PFS and ORR, these findings support the benefit of belzutifan in patients with advanced clear cell RCC after prior immune checkpoint and anti-angiogenic therapies
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Director, Barts Cancer Centre at St. Bartholomew's Hospital, London, UK
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:1. Choueiri TK, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med. 2021;27(5):802-5.
2. Albiges L, et al. LBA88 Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): Randomized open-label phase III LITESPARK-005 study. Ann Oncol 2023;34(2):S1329-S1330.
Read the Discussion: ASCO GU 2024: Insights from CheckMate 67T and Patient-Reported Outcomes in LITESPARK-005 for Advanced RCC


