(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session addressing the incorporation of radiation therapy, theranostics, and biomarkers into the management of renal cell carcinoma (RCC). Dr. Brian Shuch discussed the emergent role of theranostics in RCC.
Dr. Schuch noted that kidney cancer was falsely believed to be radioresistant for over two decades. Some of this ‘dogma/belief system’ originated from an in vitro study of 694 cell lines of which RCC/kidney fibroblasts accounted for one cell line, which was demonstrated to have the lowest sensitivity to radiotherapy. This dogma has been challenged since as ablative radiation doses now demonstrate excellent rates of short-term local control for bone, brain, lung, and soft tissue metastases and primary tumor treatment (recent data from iROCK and FASTRACK II).
Recent data from the TCGA dataset has demonstrated that subsets of RCC may harbor DNA Damage Repair (DDR) defects. DDR deficient cancers have enhanced sensitivity to systemic therapy and radiotherapy, and thus certain histologic subtypes of RCC, including clear cell, papillary, and chromophobe may have higher sensitivity to such treatments, secondary to their genomic profile.

How can we deliver radiation in kidney cancer? Stereotactic body radiotherapy (SBRT) to distant sites appears to impact metastatic RCC disease course when applied to a limited number of sites, but most RCC patients have many sites of disease. Collateral damage to adjacent tissue limits dosing and number of possible treatments. It is impossible and not feasible to plan treatment to unlimited metastatic sites. What about the primary tumor site (i.e., kidney)? Dr. Schuch noted that the dose is limited by surrounding structures (duodenum, colon, etc.). The tumor still enhances and is viable after SBRT (although markers of cellular proliferation such as Ki67 are reduced), which raises the issue of appropriate dosing. SBRT is also associated with moderate renal toxicity, with the median eGFR decreasing from 60 mL/min to 43 mL/min in FASTRACK II. Are there alternative ways to deliver ionizing radiation safely and effectively in RCC?
One potential way is brachytherapy. This is commonly performed for prostate and cervical cancers, whereby local doses of radiotherapy are released, minimizing toxicity to surrounding organs, but is clearly not feasible for multiple disease sites. As such. Dr. Schuch in collaboration with his UCLA radiation oncology colleague, Dr. Albert Chang, have opened a single arm trial evaluating interstitial brachytherapy with Iridium-192 for the treatment of ‘inoperable’ kidney cancer patients.
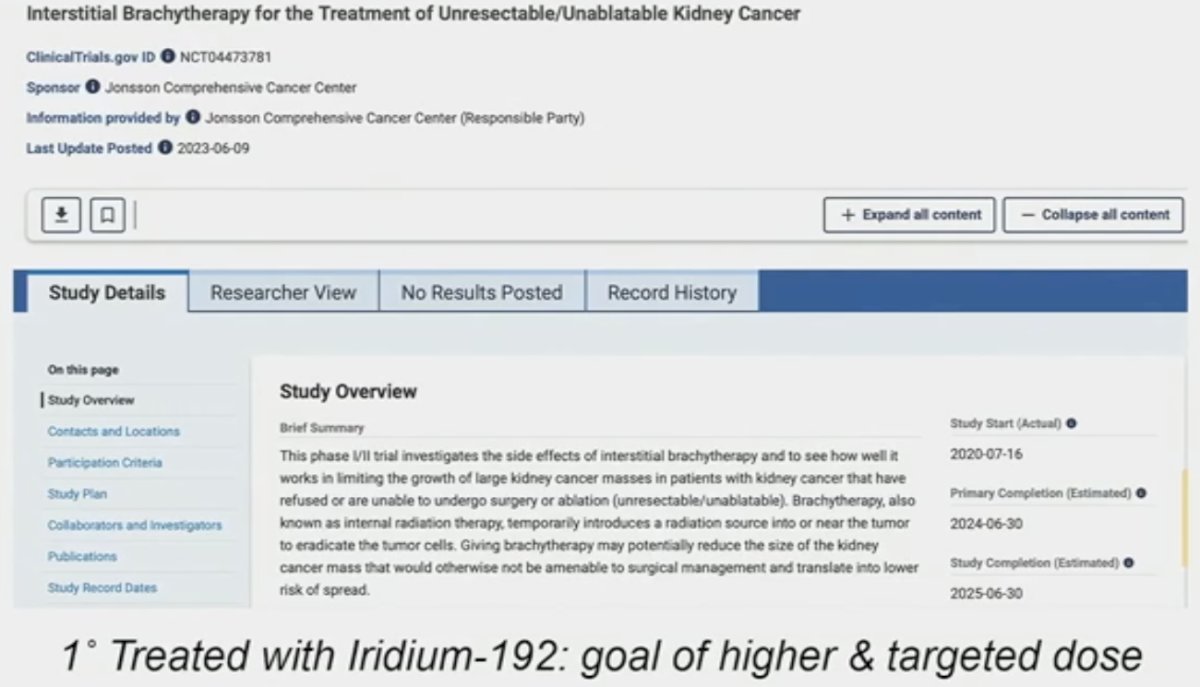
With this approach, catheters are placed with the involvement of the urology, radiation oncology, and interventional radiology teams. Some concerns with such an approach are seed migration and stone formation; however, Dr. Schuch noted that to date from their experience, these have not been issues. He gave a case example of a patient with a 6 cm hilar tumor who did not want surgical intervention. This patient received dose escalation to 54 Gy, and on repeat imaging, has no evidence of tumor enhancement, with no disease recurrence two years post-treatment. He did acknowledge, however, that such an approach is not feasible for multiple disease sites.

Another alternative delivery mechanism may be radionuclides. The foundation of radionuclides for cancer therapy dates to the 1940s with radioiodine for thyroid cancer. It is an alternative method of delivery of radiation therapy that:
- May reduce collateral damage to adjacent tissue
- May allow increased and tumoricidal doses
- May provide treatment systemically, rather than to limited areas
Radionuclides with alpha and beta decay/emission causes direct DNA damage to targeted cells.
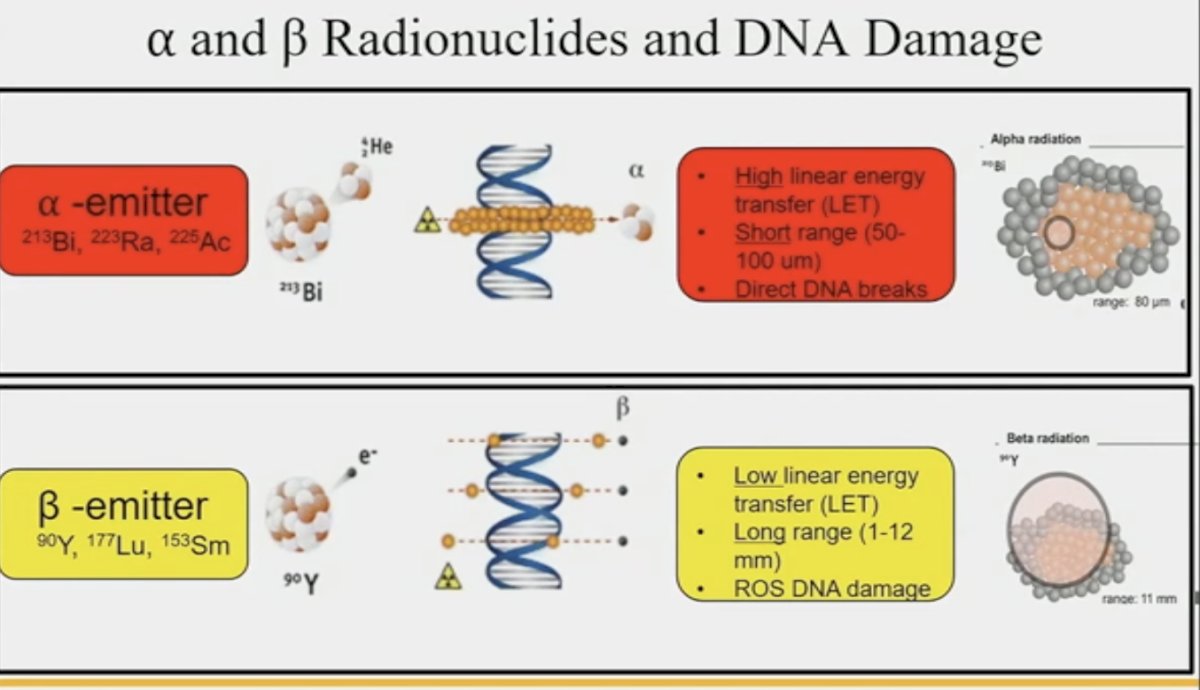
While both alpha and beta radionuclides cause DNA damage, alpha emitters cause direct damage due to high linear energy transfer, whereas beta emitters cause more indirect DNA damage through reactive oxygen species. Pancreatic models with SSRT/DOTATATE comparing 177Lu and 225Ac show both cause double-stranded DNA breaks.
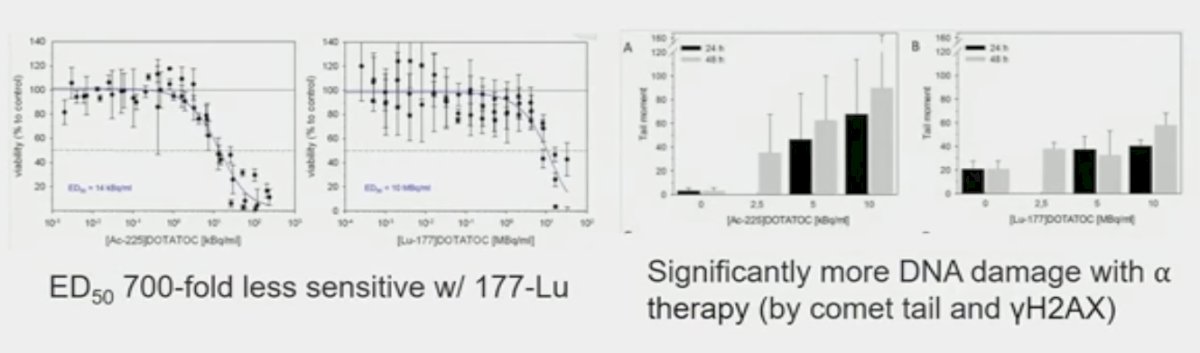
However, alpha radionuclides, including Radium and Actinium, are less readily available globally with the recent Ukrainian conflict restricting supplies of these agents, which are largely produced in The United States and Russia.
How can these radionuclides be delivered?
- Unconjugated
- Preferential organ uptake (e.g., thyroid or bone): 131I, 223Ra, 153Sm
- Direct injection into joints (radiosynovectomy)
- Nanoconstruct and microsphere (glass- or resin-based)
- Small molecule (MIBG – norepinephrine analog)
- Peptide substrates (e.g., PSMA-617 and DOTATATE)
- Antibody (e.g., J591-PSMA ab and Girentuximab to CAIX)
Microspheres can be bound to radionuclides such as Yttrium-90 (Y90) allowing for beta energy emission, which leads to very high targeted radiation doses in the range of 100 to 400 Gy (classically <100 with SBRT) when delivered into the tumor via the arterial vasculature.

There is a precedent for the use of trans-arterial Y90 radioembolization in hepatocellular carcinoma and colorectal metastases. This technology has been evaluated for the treatment of patients with liver-dominant metastatic RCC. A report from the Moffitt Cancer Center tested this approach in 18 such patients. The median dose delivered was 137.6 Gy. None of the patients had grade ≥3 toxicity and 16/17 patients had mRECIST complete responses with low rates of liver progression.1
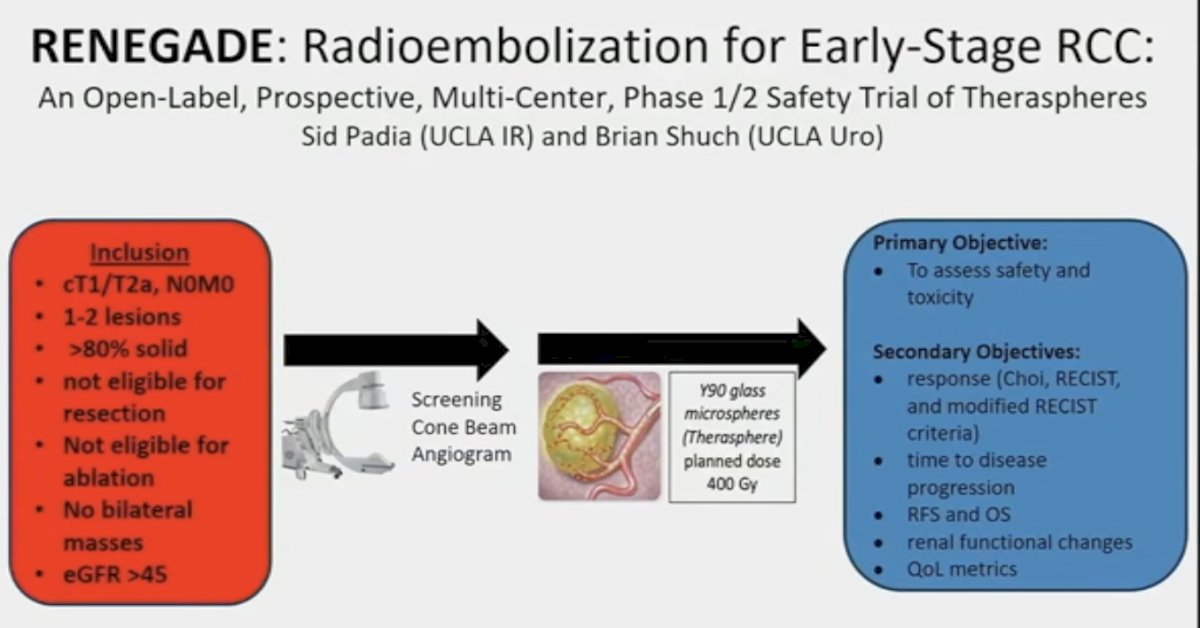
Can this approach be adopted for primary tumors? An Australian phase 1 trial (RESIRT) tested this approach with SIR spheres, whereby doses of 76 to 300 Gy were administered. The median tumor size was 5 cm, and patients were not eligible for surgery/ablation. The majority of patients had stable disease per RECIST (stable disease: 86%). There were no grade ≥3 toxicities, and patients retained excellent renal outcomes (median eGFR: 76 to 76 mL/min at 12 months).
This is the rationale for the phase 1/2 RENEGADE trial of radioembolization for early stage RCC that has been recently opened for accrual, with Dr. Schuch as one of the principal investigators
What about targeted systemic radionuclide therapy, analogous to Lu-PSMA in prostate cancer? These are diagnostic imaging agents utilized for systemic treatment (i.e., see then treat). Quantitative SPECT/CT possible with 177Lu for dosimetry (B and y decay). This approach has demonstrated success in prostate (PSMA), pheochromocytoma (MIBG), and endocrine tumors (DOTATATE). Renal imaging antibodies are available for transition.

In addition to prostate cancer cells, PSMA is expressed in tumor-associated neovasculature of the majority of renal cortical tumors (endothelial cells). These are most diffusely and intensely expressed in the tumor vessels associated with clear cell RCC. However, the expression levels are low, and it is unclear if there are expressed mostly at the surface or within the intracellular space. Previous reports of 177Lu- PSMA use in PSMA-expressing ccRCC with disease progression following nivolumab and everolimus have demonstrated poor binding specificity and rapid washout with minimal to no responses in such patients.
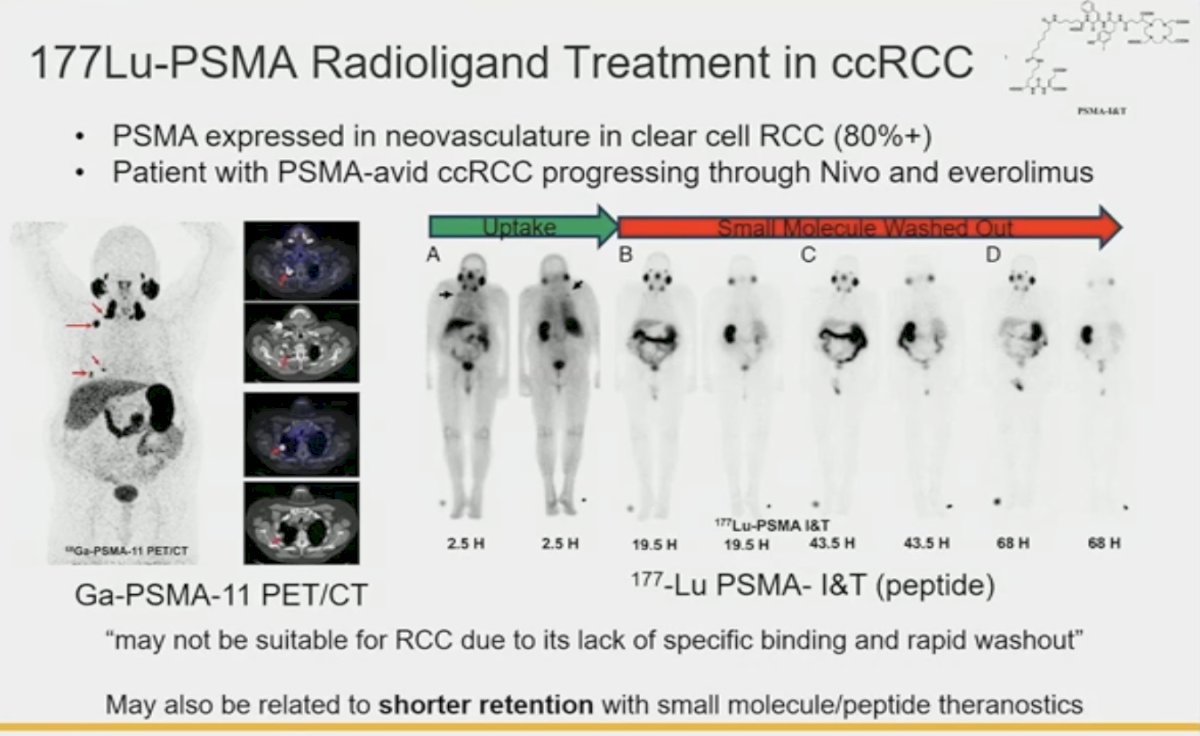
This highlights the clear need for RCC-specific cell surface targetable proteins, which led to the discovery of carbonic anhydrase IX, the “holy grail of RCC cell surface molecules”. In the phase 3 ZIRCON study presented last year, it was demonstrated that 89Zr-DFO-girentuximab for PET/CT imaging had a positive predictive value of 95% for the detection of primary tumor ccRCC in approximately 330 evaluable patients.
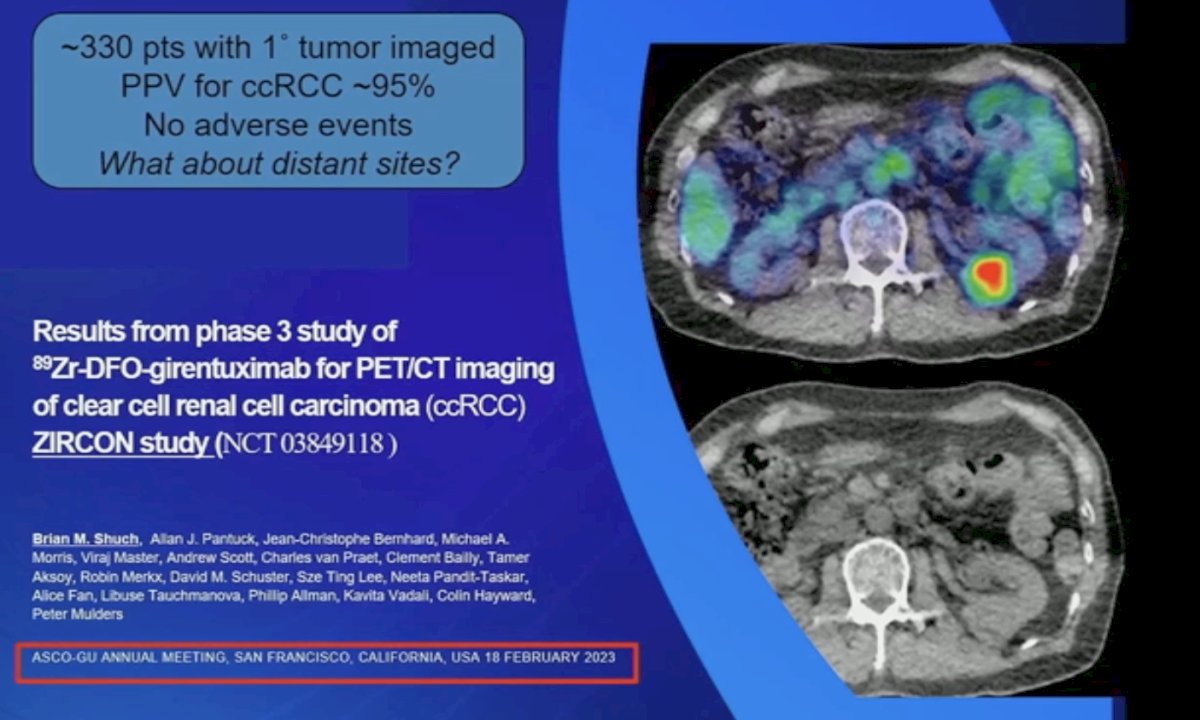
What about distant metastatic sites? The Impact-RCC multicenter prospective trial conducted in the Netherlands included 42 patients with metastatic ccRCC imaged prior to systemic therapy. Compared with [18F]FDG-PET and cross-sectional imaging, 89Zr-DFO-girentuximab PET/CT outperformed both modalities for the detection of bone and soft tissue lesions.3
Can Lutetium be combined with Girentuximab? In a phase 2 radioimmunotherapy trial with 177Lu-girentuximab in 14 patients with progressive metastatic ccRCC, 57% of patients had stable disease and one (7%) had a partial regression. The treatment was generally well tolerated but resulted in grade 3-4 myelotoxicity in most patients. This provided a ‘proof of concept’ that Lutetium could be administered to such patients with some degree of response.4

We may also observe the emergence of combination radionuclide therapy that may allow for reduced doses and circumvent resistance mechanisms. Radionuclide + DNA repair inhibitors may have possible synergy by impeding the repair of induced DNA damage. Radionuclide treatment can induce damage-associated molecular patterns (DAMPs) and tumor-associated antigens, attracting immune cells to the site, perhaps synergistically working with immunotherapy.
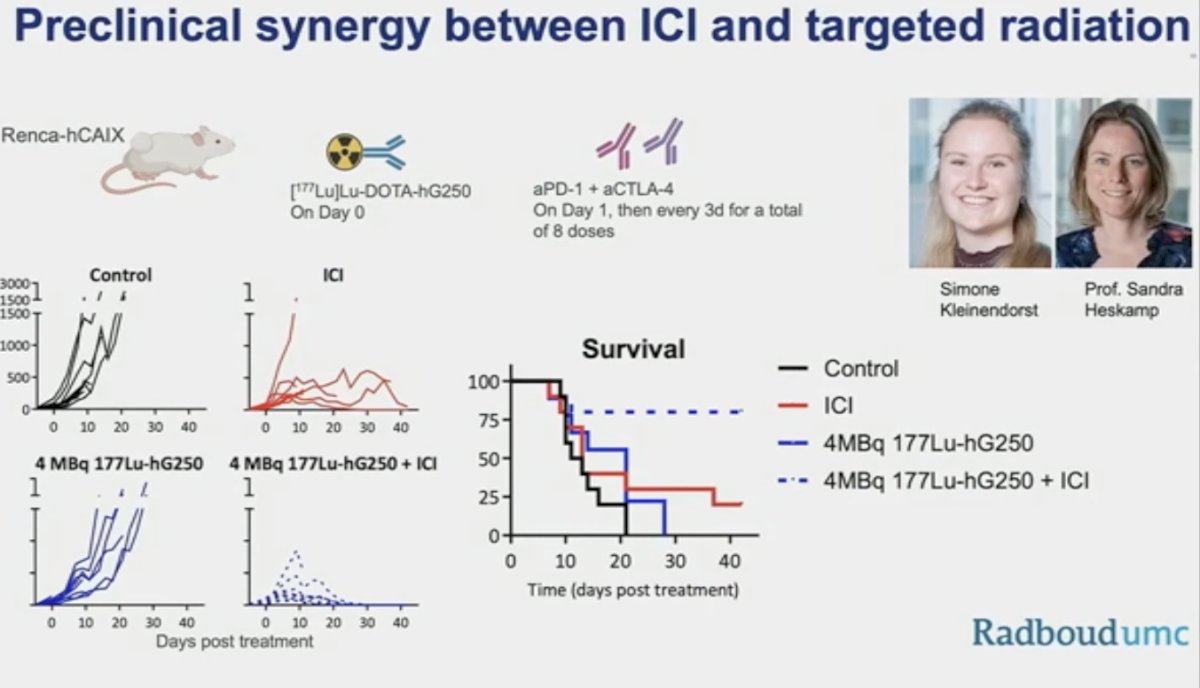
Dr. Schuch concluded as follows:
- These are exciting times for RCC clinicians, as we have immuno PET/CT for ccCC suitable for radionuclide delivery, and we may be on the cusp of a nuclear medicine revolution, similar to that observed with PSMA for prostate cancer
- RCC is not radioresistant; besides SBRT, we can deliver very high doses of radiation via alternative approaches
- Primary tumor and systemic treatment options are under investigation
- Many unanswered questions remain
- Dose, frequency, monotherapy versus combination therapy
- Use of alpha versus beta emitters
Presented by: Brian M. Shuch, MD, Associate Professor, Department of Urology, University of California, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Kis B, et al. Transarterial Yttrium-90 Radioembolization Treatment of Patients with Liver-Dominant Metastatic Renal Cell Carcinoma. J Vasc Interv Radiol. 2017;28:254-259.
- De Souza PL, et al. RESIRT: A Phase 1 Study of Selective Internal Radiation Therapy Using Yttrium-90 Resin Microspheres in Patients With Primary Renal Cell Carcinoma. Clin Genitourin Cancer. 2022;20(5):442-451.
- Verhoeff SR, van Es SC, Boon E, et al. Lesion detection by [89Zr]Zr-DFO-girentuximab and [18F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46(9):1931-1939.
- Muselaers CHJ, Boers-Sonderen MJ, van Oostenbrugge TJ, et al. Phase 2 Study of Lutetium 177-Labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur Urol. 2016;69(5):767-770.


