(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a renal cell, adrenal, and testicular cancers rapid oral abstract session. Dr. Reuben Ben-David presented the results of an analysis of a longitudinal tumor-informed circulating tumor DNA (ctDNA) assay and patient outcomes in testicular cancer.
Serum-based tumor marker, namely alpha-fetoprotein, β-HCG, and LDH are currently utilized for the surveillance of testicular cancer patients; however, these markers lack sufficient sensitivity and specificity for the detection of molecular residual disease. ctDNA holds promise as a prognostic biomarker across various malignancies. Yet, its clinical utility in testicular cancer remains underexplored. The study objective was to evaluate the utility of longitudinal ctDNA monitoring as a reliable prognostic marker in testicular cancer patients.
A total of 145 plasma samples were collected from 35 patients with stages I to Ill testicular cancer. Longitudinal cDNA testing was performed using a personalized, tumor-informed cDNA assay (Signatera™ bespoke mPCR-NGS assay) that has been clinically validated across multiple tumor types. ctDNA was evaluated during both the ‘molecular residual disease’ (1-12 weeks post-orchiectomy) and surveillance period (>12 weeks post-orchiectomy, post-adjuvant chemotherapy, or post-retroperitoneal lymph node dissection [RPLND]) windows. The correlation between ctDNA status and event-free survival (EFS) was assessed. EFS was defined as the interval from radical orchiectomy to the date of radiological recurrence or any evidence of residual/persistent disease after the completion of adjuvant chemotherapy or RPLND.
The baseline patient characteristics of the 35 study patients are summarized below. Notably, 43% underwent surveillance, 23% chemotherapy alone, 9% RPLND, and 26% RPLND and chemotherapy. Pre-operative serum tumor markers were elevated in 79% of patients, and 49% had elevations anytime post-operatively. The total follow-up duration was 10 months, and the event-free survival was 8 months.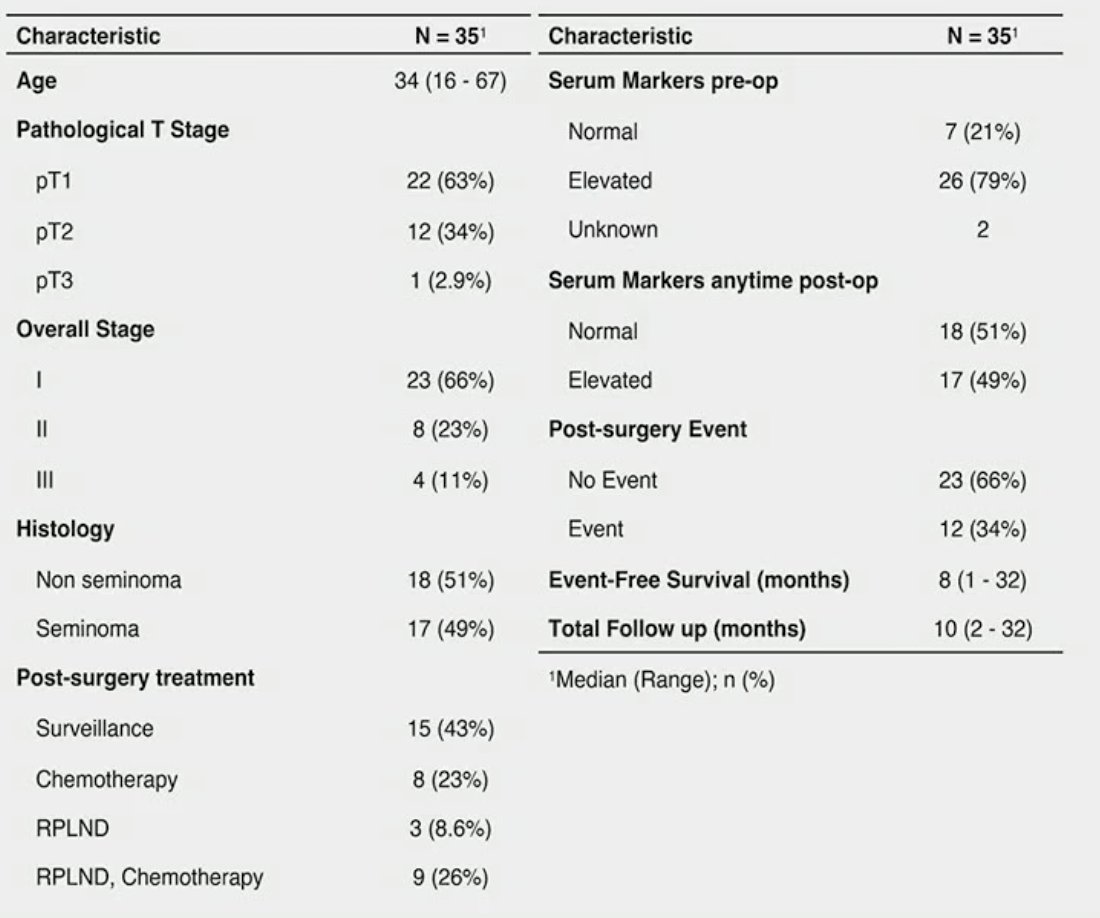
In the flow chart below, the incidence of positive ctDNA during the pre-orchiectomy, molecular residual disease, surveillance, and anytime post-orchiectomy windows are summarized below: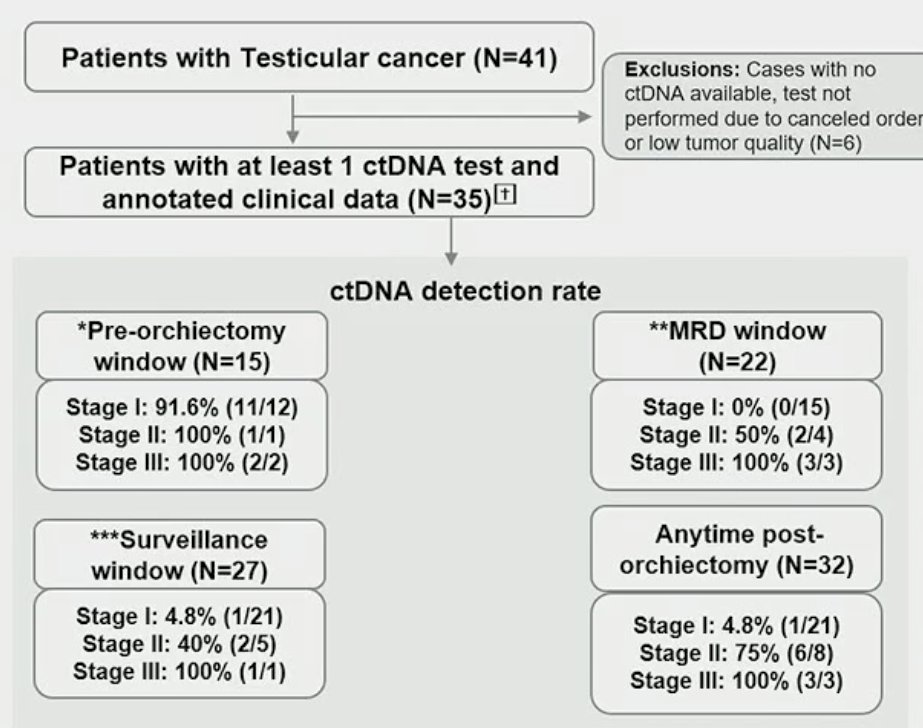
ctDNA proved to be a prognostic marker in the molecular residual disease window (i.e., 1 – 12 weeks post-orchiectomy), with the 4 ctDNA positive patients having a 12-months EFS of 25% only, compared to 76.5% who were ctDNA during this window.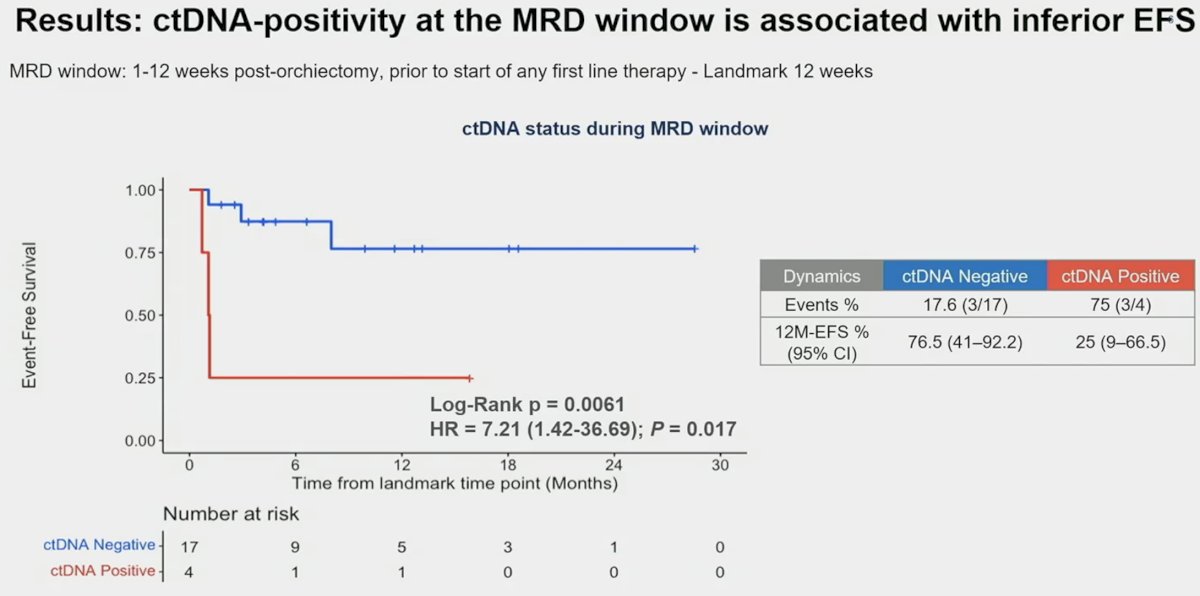
Similarly, a positive ctDNA status during the surveillance window was associated with significantly worse EFS (0% versus 87%) among the 3 patients with a positive ctDNA.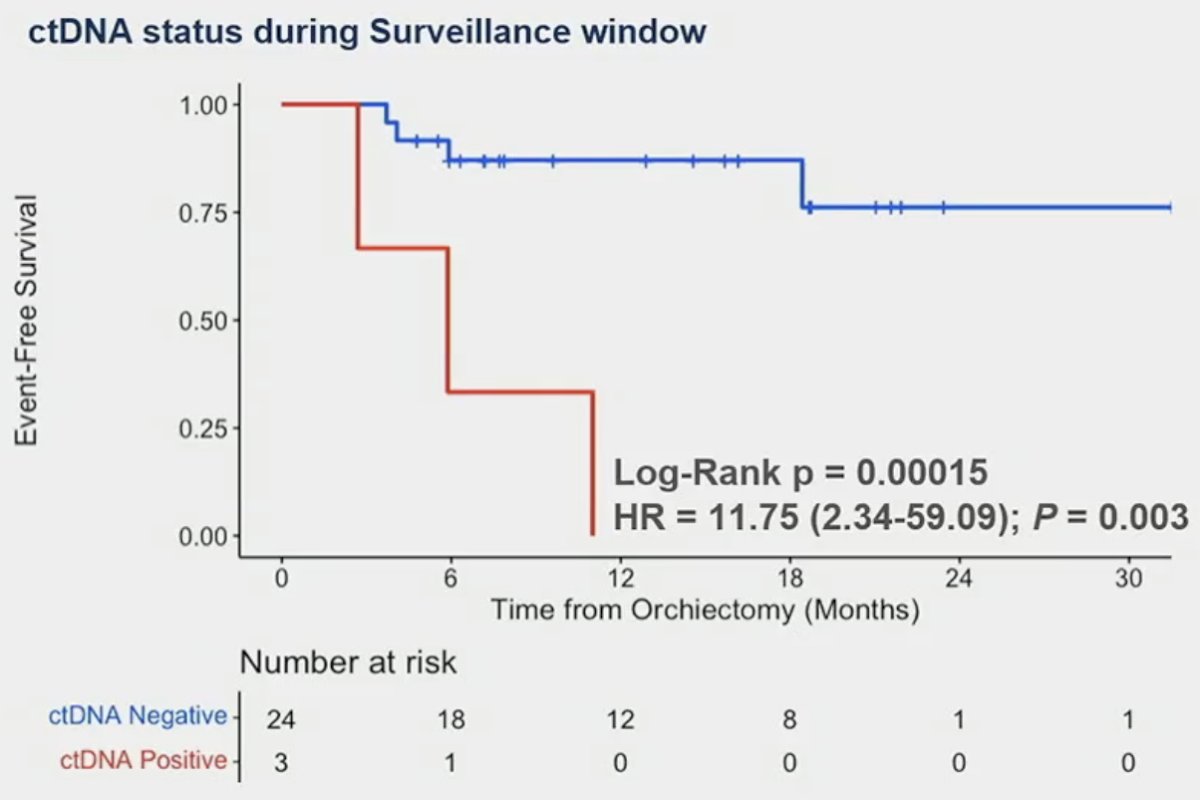
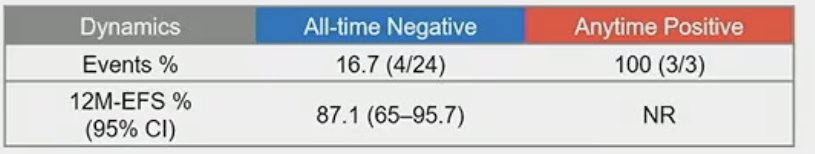
What about the correlation between ctDNA status and serum tumor markers? Among patients with normal markers, 2/21 had a positive ctDNA, and both of these patients relapsed. Conversely, among the 3 patients with a positive ctDNA status during surveillance, 2/3 had normal markers.
Dr. Ben-David concluded that:
- This is one of the first reports that utilizes longitudinal tumor-informed ctDNA testing to assess clinical outcomes and disease status in patients with testicular cancer.
- cDNA status during the molecular residual disease and surveillance windows is significantly associated with event-free survival in patients with testicular cancer.
- Future prospective studies are needed to assess the utility of ctDNA in patients with testicular cancer.
Presented by: Reuben Ben-David, MD, Society of Urologic Oncology Fellow, Icahn School of Medicine at Mount Sinai, New York, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


