(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a renal cell, adrenal, and testicular cancers rapid oral abstract session. Dr. Rebecca Hassoun presented the results of an analysis evaluating the utility of circulating tumor DNA (ctDNA) as a predictive biomarker for disease monitoring in patients with non-seminomatous germ-cell tumors.
Serum tumor markers including AFP, β-hCG, and, to a lesser extent, LDH, are currently utilized for the diagnosis and management of non-seminomatous germ cell tumors (NSGCT). Serum tumor markers are normal in a substantial proportion of patients with NSGCT and can be falsely elevated in certain clinical conditions – thus lacking optimal sensitivity and specificity. Limitations in these performance characteristics create an unmet need for early detection of molecular residual disease in order to accurately predict clinical outcomes at earlier stages. In this study, Dr. Hassoun and colleagues evaluated the clinical utility of ctDNA testing for predicting clinical outcomes.
This study included 25 patients with stage II and III NSGCT, with a total of 106 plasma samples collected. Longitudinal ctDNA testing was performed using a personalized, tumor-informed ctDNA assay (Signatera™ bespoke mPCR-NGS assay) that has been clinically validated across multiple tumor types. ctDNA results were analyzed and evaluated for their correlation with event-free survival. EFS was defined as the interval from orchiectomy to the date of recurrence or evidence of residual/persistent disease after the completion of Retroperitoneal lymph node dissection (RPLND) or chemotherapy.
The baseline patient characteristics are summarized below. The median age was 29 years. The primary treatment for the stage II-III patients was chemotherapy for 68%, chemotherapy + RPLND for 20%, and RPLND for 12%. The pre-orchiectomy serum tumor markers were elevated in 56% of patients and were elevated during the disease course in 64% of patients.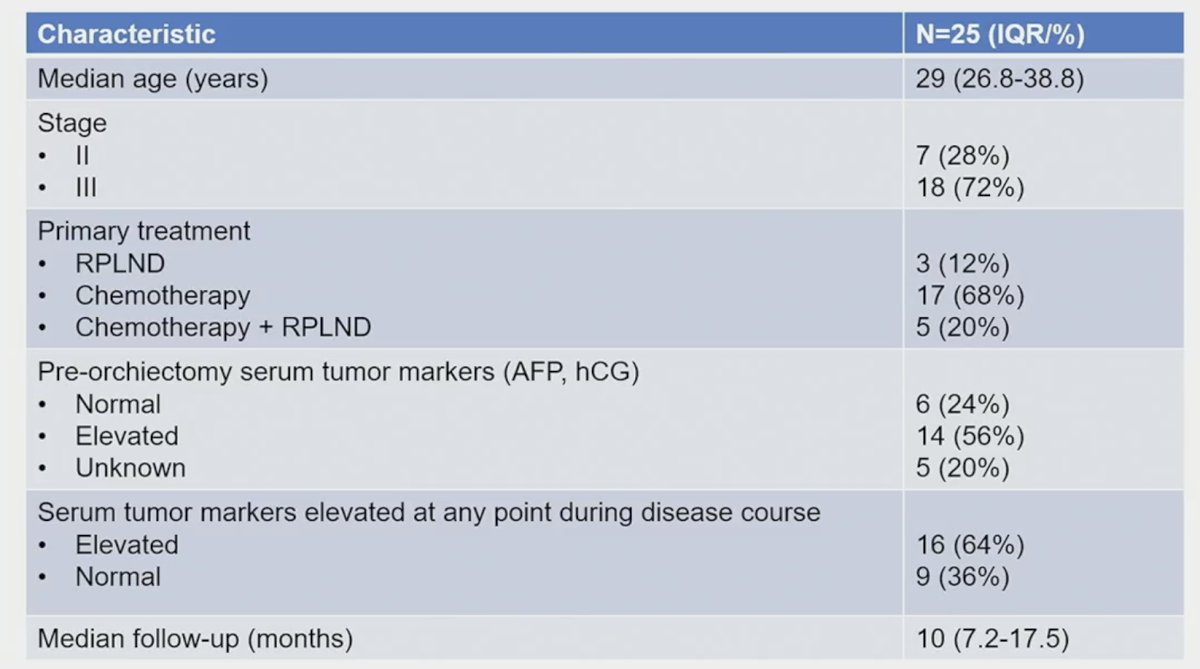
The schematic below illustrates the ctDNA detection rates during the follow-up windows. During the early molecular residual disease window, 5/7 patients had positive ctDNA (all stage III), while 5/11 patients in the surveillance window had positive ctDNA.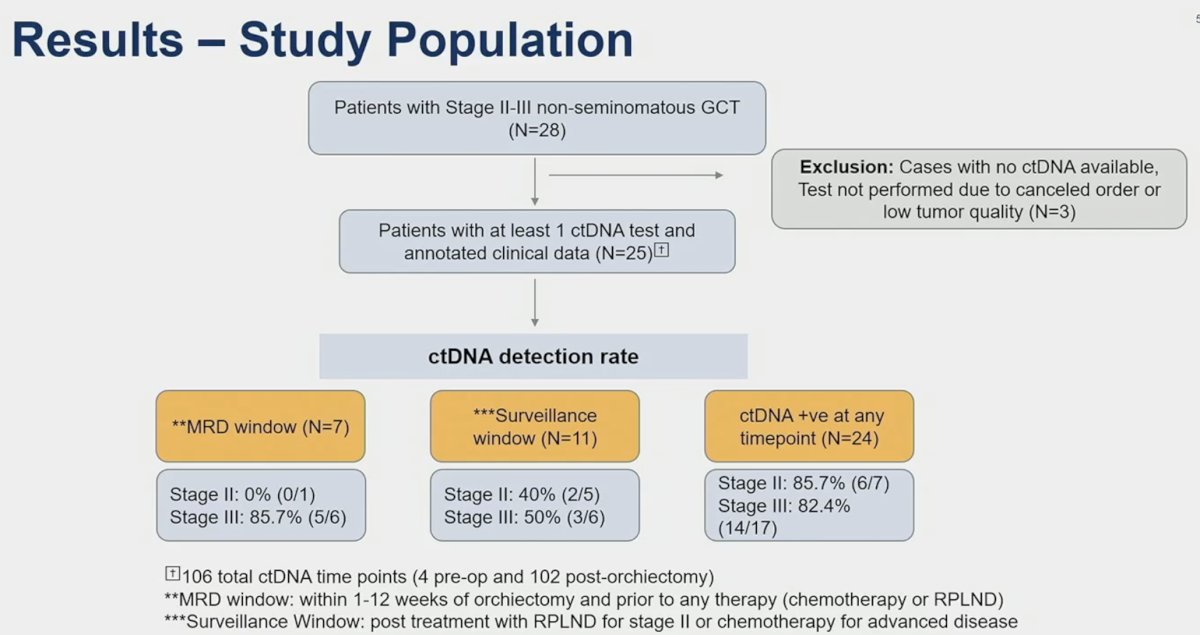
As demonstrated below, ctDNA outperformed serum tumor markers as a prognostic biomarker of event-free survival post-RPLND for stage II patients and post-chemotherapy for advanced disease. Compared to patients with a negative ctDNA status, those who were ctDNA positive had an 11.4-fold increased hazard of an event (p-0.029), compared to an HR of 2.14 when comparing elevated to normal serum tumor markers. At a median follow-up of 10 months, none of the six patients with a negative ctDNA status had an event.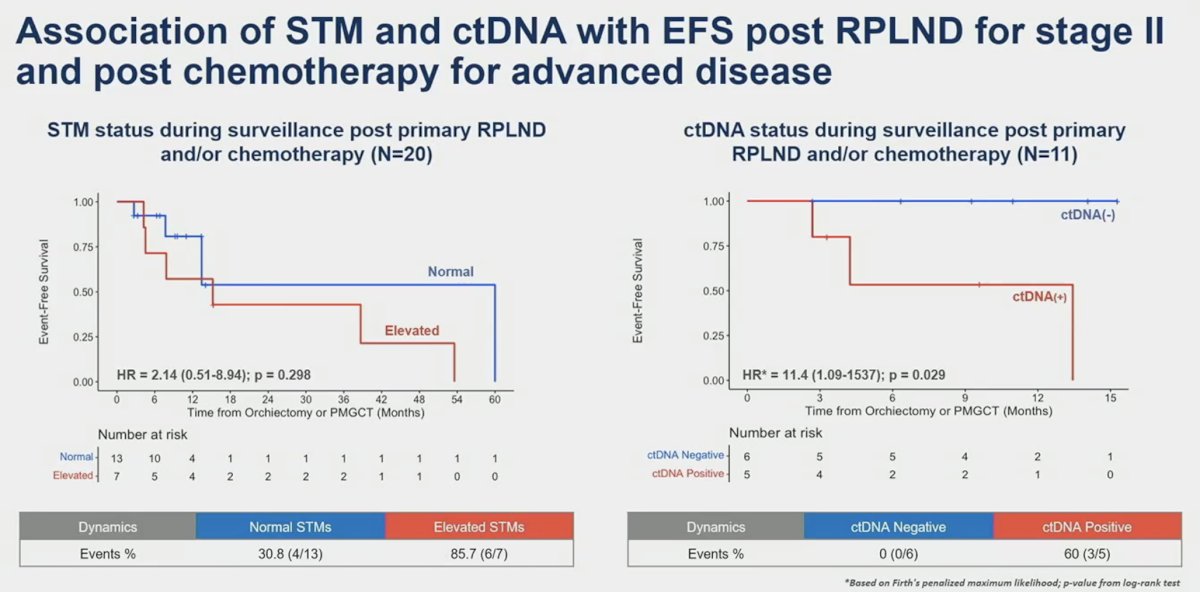
Dr. Hassoun concluded that:
- This is one of the first reports outlining the clinical implications of cDNA monitoring in patients with non-seminomatous GCT
- ctDNA status post-primary RPLND and/or chemotherapy was significantly associated with EFS in patients with NSGCT
- Personalized monitoring of ctDNA seems prognostic of recurrence in patients with non-seminomatous GCT
- Larger prospective studies are needed to validate the findings of this study
Presented by: Rebecca Hassoun, MD, Postdoctoral Fellow in Medicine, Indiana University Simon Comprehensive Cancer Center, Indiana, IN
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


