(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer poster session. Dr. Umang Swami presented the stage 1 results of a phase 2 trial evaluating enfortumab vedotin as monotherapy in patients with metastatic castration-resistant prostate cancer (mCRPC).
Enfortumab vedotin is an antibody-drug conjugate consisting of a fully humanized monoclonal antibody directed against the extracellular domain of Nectin-4, conjugated to a microtubule-disrupting agent, monomethyl auristatin E (MMAE) via a protease-cleavable linker. While enfortumab vedotin has been approved as a single agent treatment option for metastatic urothelial carcinoma patients with disease progression following platinum-based chemotherapy and immunotherapy (EV-301)1 and in combination with pembrolizumab for patients with locally advanced/metastatic urothelial carcinoma, irrespective of cisplatin eligibility,2 animal models of prostate cancer metastatic lesions have also shown high membranous Nectin-4 expression (PMID: 30767361). Accordingly, Dr. Swami and colleagues aimed to investigate this antibody-drug conjugate in patients with heavily pre-treated mCRPC.
This is an investigator-initiated, single-arm, single-center, phase 2 trial of enfortumab vedotin (1.25 mg/kg IV), administered on days 1, 8, and 15 of 28 days cycle. The key eligibility criteria were as follows:
- ≥18 years age
- mCRPC with histologically/cytologically confirmed adenocarcinoma without small cell histology
- Performance status ≤ 1
- Adequate organ function
- Prior treatment with ≥3 cycles of docetaxel and ≥1 androgen-receptor pathway inhibitor
- Received/refused all therapies shown to improve overall survival, except cabazitaxel which is exclusionary.
The primary objective was to assess anti-tumor activity of enfortumab vedotin, as determined by one of the following:
- Objective response by RECIST 1.1 in those with measurable disease
- Confirmed conversion of circulating tumor cell count (CTC) to <5/7.5 mL blood
- PSA decline of ≥ 50%
- stable disease for ≥ 6 months per PCWG3 modified RECIST 1.1.

The investigators utilized a Simon’s two-stage design. The null hypothesis (H0) was that the true response rate is 25%, tested against a one-sided alternative. In stage I, if ≤3 responses were observed in 11 pts, the study would be stopped. Otherwise, 23 additional patients would be accrued, for a total of 3 of 4 patients. The null hypothesis would be rejected if ≥13 responses were observed in 34 patients (type I error rate 0.046, power 0.854 when true response rate is 50%).
In this presentation, Dr. Swami presented the results of the first 11 patients from the 1st stage of the study. All patients were non-Hispanic, White with a median age of 72 years (range 43 – 78). The median number of prior systemic therapies received, in addition to castration, was 5 (range: 3 – 8). 4/11 patients had RECIST measurable disease, and 7/11 had CTC ≥5 at baseline.
At a median follow-up of 9 months (range: 1 – 13), 7/11 (64%) patients had a protocol-defined response (5/11 PSA 50% responses, 3/4 objective response, 4/7 confirmed CTC response, and 2/11 ≥6 months on therapy). Of the 9 patients who discontinued treatment, 8 were due to disease progression (one withdrew consent). The median number of completed cycles was 6 (range: 2 – 12), and the median radiographic progression-free survival was 5.5 months (95% CI: 5.3 – not reached).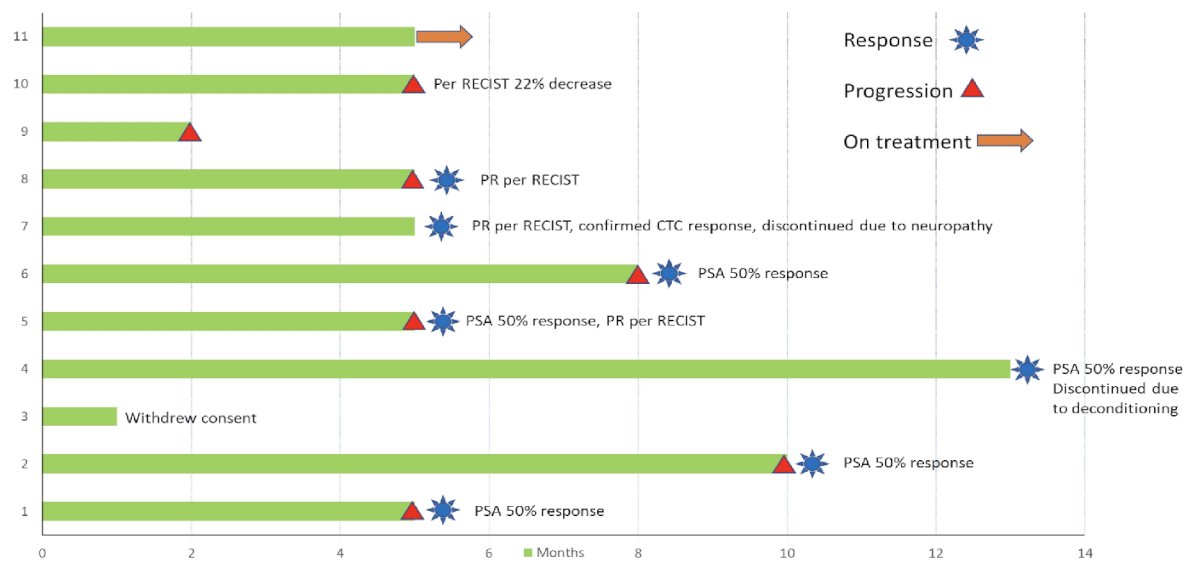
The most common grade 3-4 treatment-related adverse events were anemia, AST increase, dry skin, fatigue, hyperglycemia, rash, leucopenia (one patient each), and nausea and decrease in neutrophil count (two patients each). Eight patients required dose interruptions and/or reductions. There were no Grade 5 treatment-related adverse events.
Dr. Swami concluded that enfortumab vedotin shows promising efficacy and an acceptable safety profile in patients with heavily treated, refractory mCRPC. Further enrollment in stage 2 of the trial is ongoing.
Presented by: Umang Swami, MD, MS, Assistant Professor, Division of Oncology, Department of Internal Medicine, Huntsman Cancer Institute at the University of Utah, Salt Lake City, UT
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.
- FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer. Accessed on January 25, 2024.


