(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium featured a prostate cancer session and a presentation by Dr. Oliver Sartor discussing safety outcomes in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with radium-223 following external beam radiation therapy in the REASSURE study. The Phase 3 ALSYMPCA trial,1 was a phase 3, randomized, double-blind, placebo-controlled trial that randomized 921 patients in a 2:1 fashion to receive six injections of radium-223 or matching placebo. The primary endpoint was overall survival and patients receiving radium-223 had significantly improved median overall survival (14.9 versus 11.3 months; HR 0.70, 95% CI 0.58 to 0.83). Additionally, the safety profile was also reasonable for radium-223.
External beam radiation therapy to bone metastases is a highly effective therapy for the alleviation of bone pain in symptomatic patients with mCRPC, However, with longer follow-up, external beam radiotherapy has shown an increased risk of developing a second primary malignancy. REASSURE (NCT02141438) is a global, prospective, single-arm, observational study of radium-223 use in patients with mCRPC with bone metastases within routine clinical settings. The study design for REASSURE is as follows:

Utilizing data from the second planned interim analysis, Dr. Sartor and colleagues evaluated safety outcomes in patients with mCRPC treated with radium-223 following external beam radiation therapy in the United States.
In this descriptive analysis (data cutoff March 20, 2019), the authors included patients who received external beam radiation therapy to bone prior to radium-223 (≤2 years prior to radium-223 first dose. These results are relative to the United States subset of patients enrolled in REASSURE. The focus of this presentation is on the incidence of hematological toxicities, bone fractures, and second primary malignancies.
Among 498 patients in the United States subset, 118 patients received prior external beam radiation therapy to the bone with a summary of the baseline characteristics as follows:
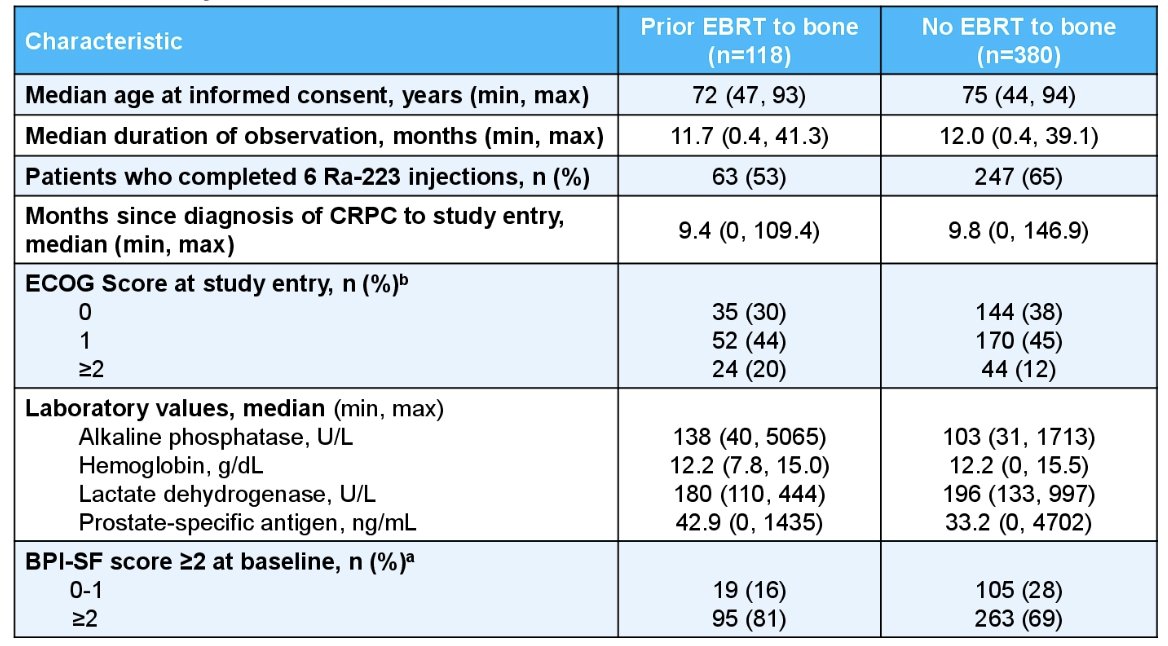
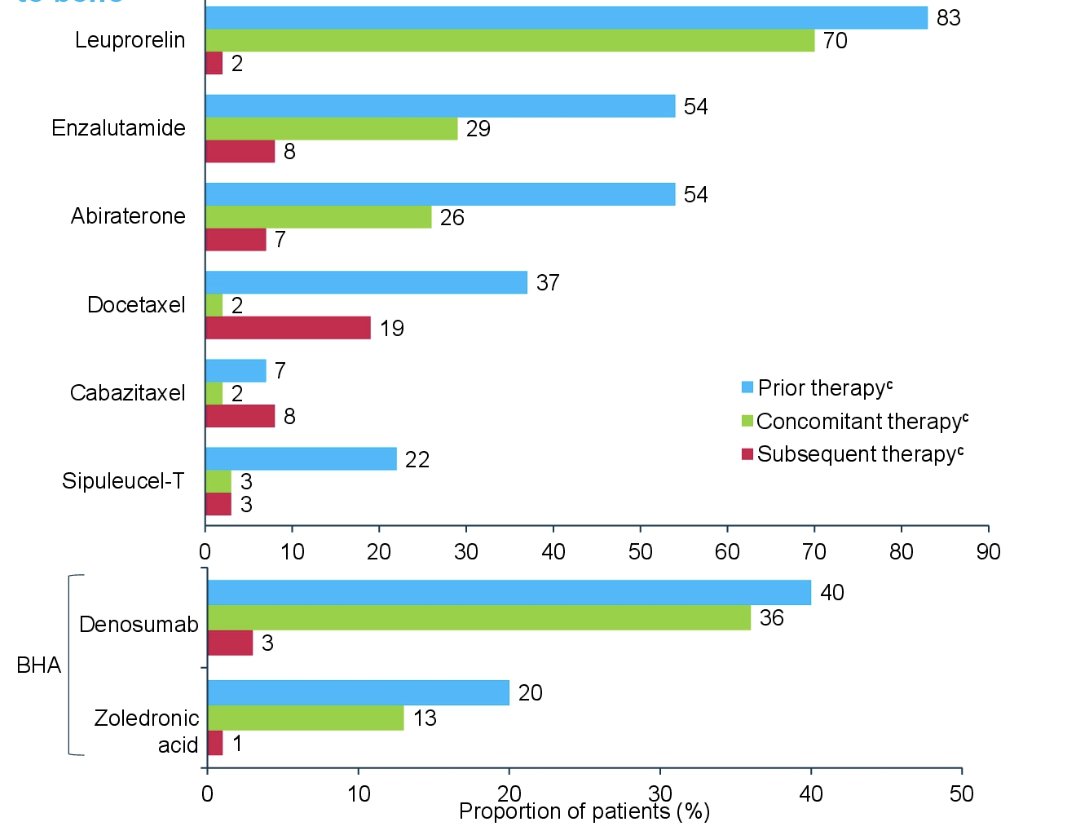
The median duration of observation was 11.7 months among patients who received external beam radiation therapy to the bone and 11.9 months for the overall US subset. Below are the anti-cancer therapies and bone health agents used among patients who received external beam radiotherapy to the bone:
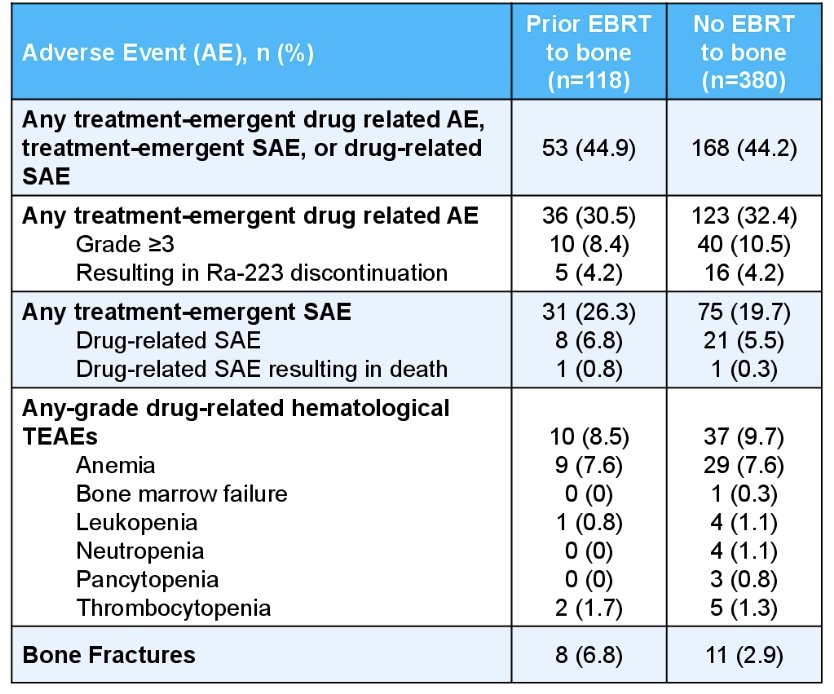
Any-grade treatment-emergent, drug-related adverse event, treatment-emergent adverse events, drug-related symptomatic adverse events occurred in 44.9% and 44.2% of patients who received external beam radiation therapy and the overall subset, respectively. Grade ≥3 drug-related hematological treatment-emergent adverse events occurred in 8.4% and 10.5% of patients who received external beam radiation therapy and the overall subset, respectively. Additionally, bone fractures occurred in 6.8% and 2.9% of patients who received external beam radiation therapy and the overall subset, respectively:
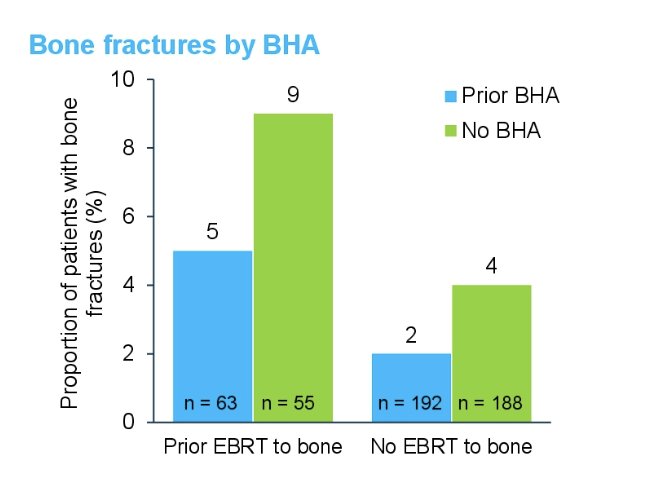
Bone fractures stratified by +/- external beam radiotherapy to the bone and +/- bone health agent were as follows:
Six second primary malignancies occurred in 5 of 118 patients (4%) who received prior external beam radiation therapy to the bone, including the gastrointestinal tract, lung, liver, skin, and a neuroendocrine tumor. There were five-second primary malignancies that occurred in patients that did not have external beam radiotherapy to the bone (1%).
Dr. Sartor concluded his presentation by discussing safety outcomes in patients with mCRPC treated with radium-223 following external beam radiation therapy in the REASSURE study with the following take-home points:
- Although concurrent external beam radiotherapy to the bone was used to treat bone pain in ALSYMPA, only a few patients among the overall US subset received radium-223 and concomitant external beam radiotherapy to the bone
- Patients who received external beam radiotherapy to the bone within 2 years prior to radium-223 did not demonstrate an increased incidence of hematological toxicities relative to patients who did not receive external beam radiotherapy to the bone
- Although the percentage of second primary malignancies was higher among patients receiving external beam radiotherapy to the bone relative to those that did not, the overall incidence remained low, and there were no hematological malignancies
- Despite a higher rate of bone fractures for patients with prior external beam radiotherapy to the bone, the overall incidence was low
- Patients treated with bone health agents had a lower number of bone fractures even among those treated with external beam radiotherapy to the bone prior to radium-223
Presented by: Oliver Sartor, MD, Mayo Clinic, Rochester, MN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
1. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.


