(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Neal Shore presented the result of a post-hoc analysis of PROpel evaluating the efficacy of combination olaparib plus abiraterone acetate/prednisone versus placebo plus abiraterone acetate/prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) and single homologous recombination repair (HRR) gene mutations.
PROpel is a global, randomized, double-blind phase 3 trial of abiraterone and olaparib versus abiraterone and placebo in patients with mCRPC treated in the first-line setting. Patients in PROpel were enrolled irrespective of HRRm status. Patients were randomized (1:1) to receive abiraterone (1000 mg once daily) plus prednisone/prednisolone with either full dose olaparib (300 mg twice daily) or placebo. The primary endpoint was imaging-based progression-free survival by investigator assessment. The trial design for PROpel is as follows:
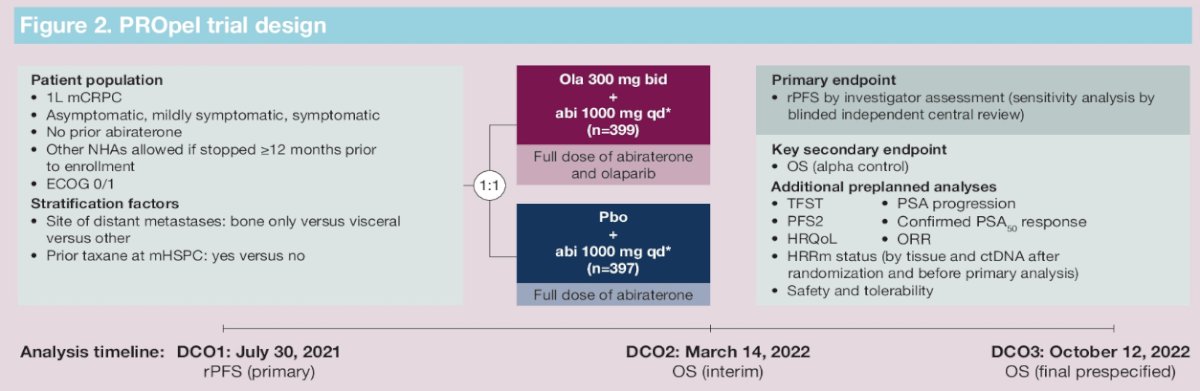
PROpel randomized 399 and 397 patients to the intervention and control arms, respectively. Median age was 70 years, with a median PSA of 17 – 18 ng/ml, and approximately 25% had received prior docetaxel. HRRm status was positive in 28% and 29% of patients in the intervention and control arms, respectively, with HRRm status unknown in 2.3%. BRCA1/2 mutations were observed in 11.8% and 9.6% of patients, respectively.
This trial met its primary endpoint, with the initial report at a median follow-up of 19.4 months demonstrating that the combination of olaparib + abiraterone significantly improved median investigator-assessed rPFS from 16.6 to 24.8 months (HR: 0.66, 95% CI: 0.54 – 0.81, p<0.001).1
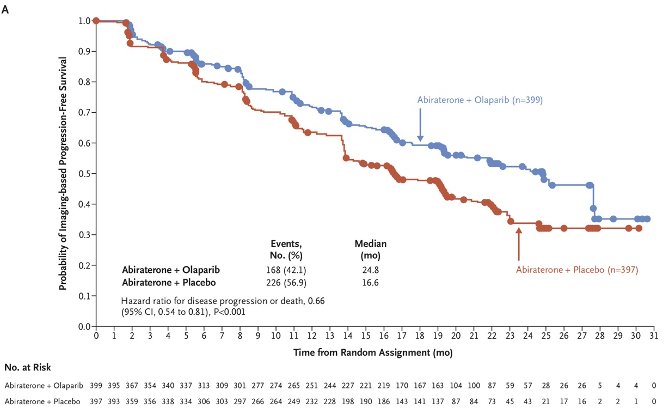
In an updated report at a median follow-up of 36.6 months, the median overall survival was 42.1 months with olaparib plus abiraterone and 34.7 months with placebo plus abiraterone (HR: 0.81, 95% CI: 0.67 – 1.00; p=0.054).2
In this report, Dr. Shore and colleagues reported the gene-by-gene efficacy of olaparib + abiraterone versus placebo + abiraterone for PROpel patients with an HRR mutation. Notably, HRRm status was assessed following randomization and before the primary analysis by tumor tissue (FoundationOne CDx) and ctDNA (FoundationOne Liquid CDx) testing and was reported using aggregated results from both tests. The genes assessed were:
- ATM
- BRCA1 & BRCA2
- BARD1
- BRIP1
- CDK12
- CHEK1 & CHEK2
- FANCL
- PALB2
- RAD51B, RAD51C, RAD51D, and RAD54L.
From a statistical analysis standpoint, Dr. Shore noted that hazard ratios and confidence intervals were not reported in subgroups with <5 events in either arm for both rPFS and OS.
Overall, 28.4% of patients had an HRR mutation.

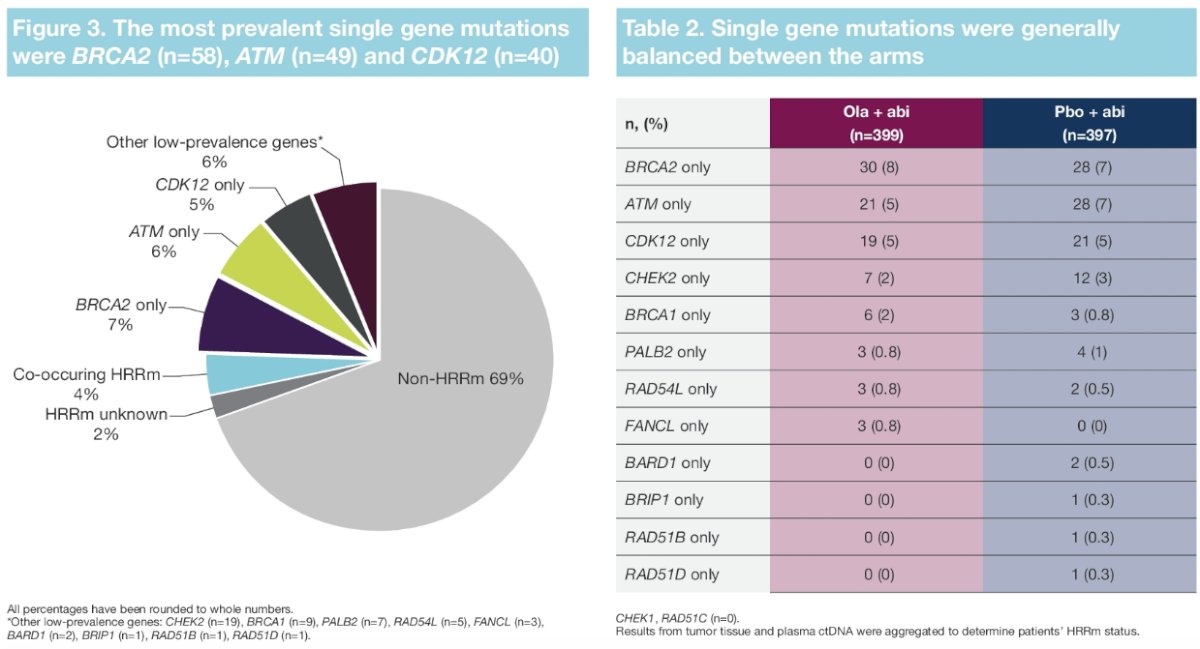
For most patients with a single gene HRR mutation, there was both an rPFS and OS benefit to the addition of olaparib to abiraterone. The most prevalent gene mutations were BRCA2, ATM, and CDK12. The rPFS outcomes for patients with these gene mutations were as follows (HR <1 favors the olaparib + abiraterone combination):
- BRCA2: HR=0.20, 95% CI= 0.08 – 0.44
- ATM: HR=0.55, 95% CI= 0.20 – 1.38
- CDK12: HR=0.51, 95% CI= 0.20 – 1.18
The corresponding OS outcomes were as follows:
- BRCA2: HR=0.20, 95% CI= 0.07 – 0.48
- ATM: HR=0.79, 95% CI= 0.33 – 1.77
- CDK12: HR=0.57, 95% CI= 0.24 – 1.27
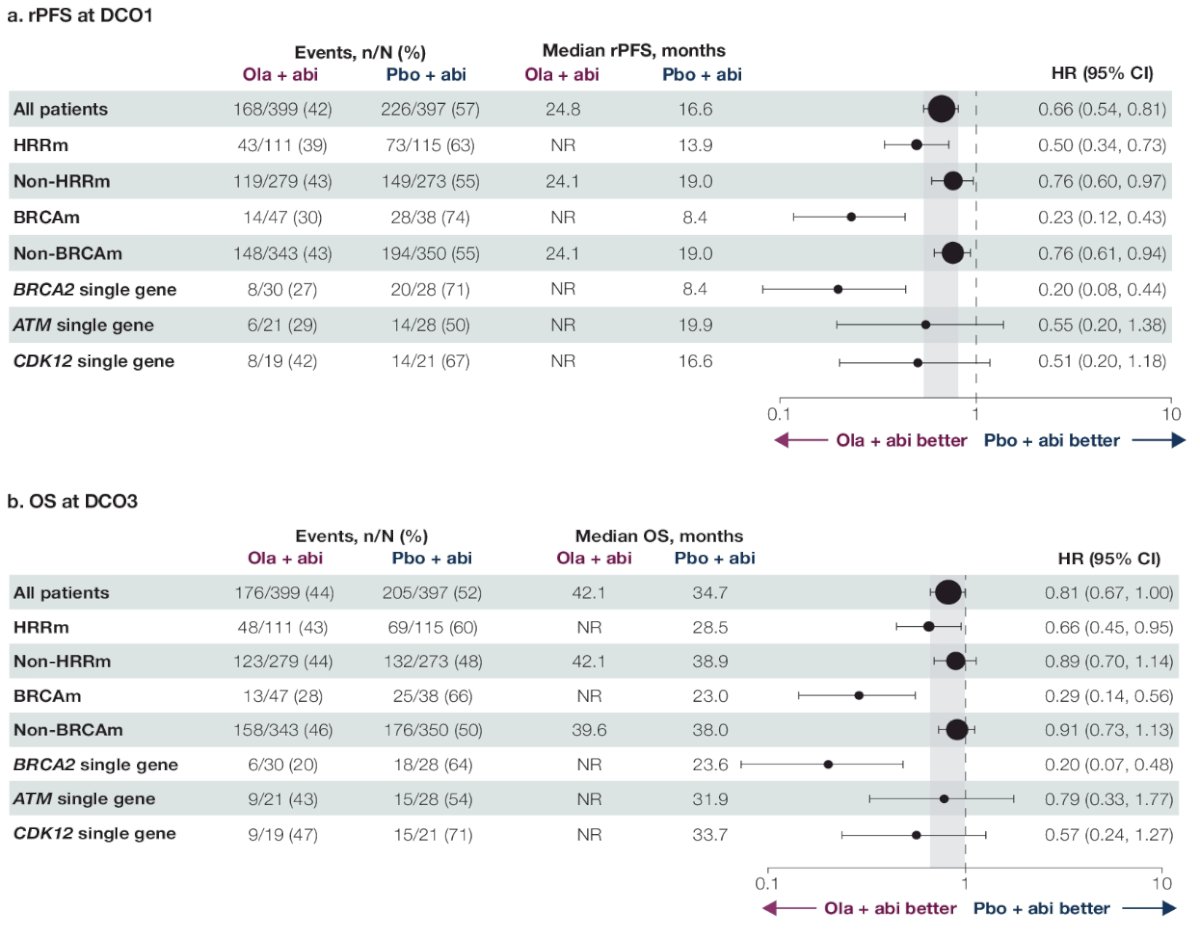
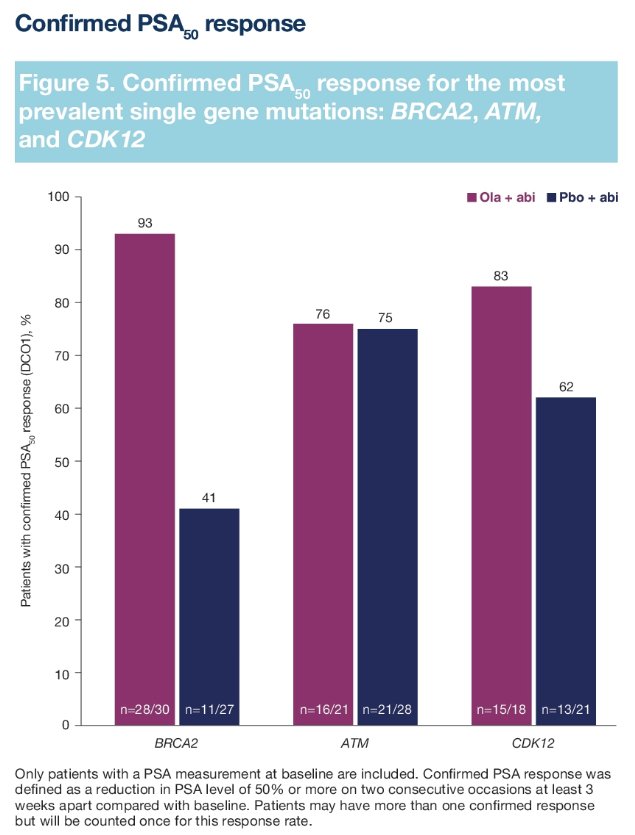
Dr. Shore concluded that BRCA2, ATM, and CDK12 were the most prevalent single gene mutations, and mCRPC patients harboring these mutations derived both a clinical benefit from the addition of olaparib to abiraterone acetate. Other single gene mutations were rare, limiting interpretation. The greatest treatment benefit was observed in patients with BRCA mutations.
Presented by: Neal Shore, MD, FACS, Urologist, Director, CPI, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SCWritten by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence 2022.EVIDoa2200043.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.


