(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Neal Shore presented the results of a post-hoc, sensitivity analysis from ARASENS accounting for subsequent therapy received, to evaluate the overall survival (OS) benefit with darolutamide versus placebo, in combination with androgen deprivation therapy plus docetaxel, for patients with metastatic hormone sensitive prostate cancer (mHSPC).
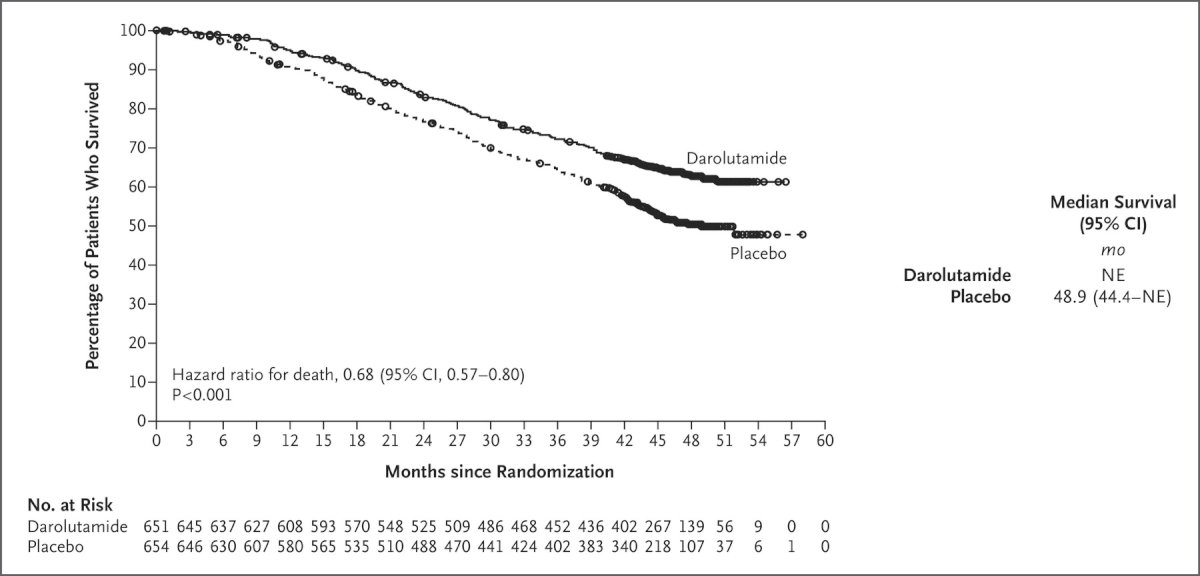
ARASENS is an international, double-blind, phase 3 trial that randomized 1,306 mHSPC patients between November 2016 and June 2018 in a 1:1 fashion to darolutamide 600 mg twice daily or matching placebo in addition to ‘standard of care’ therapy with ADT plus docetaxel. 85% of patients had de novo metastatic disease. The primary analysis demonstrated that patients in the triplet darolutamide arm had a 32.5% lower rate of death (HR: 0.675, 95% CI: 0.57 – 0.80, p<0.001), despite 76% of patients in the placebo arm receiving subsequent life-prolonging therapy.1
An updated report published in 2023 demonstrated that darolutamide improved OS, compared to placebo, in patients with high-volume (HR: 0.69, 95% CI: 0.57 – 0.82), high-risk (HR: 0.71, 95% CI: 0.58 – 0.86), and low-risk disease (HR: 0.62, 95% CI: 0.42 – 0.90).2
To address the impact of informative intercurrent events (e.g., use of subsequent therapy) in censored patients, as defined by the European Medicines Agency, Dr. Shore and colleagues performed a post hoc sensitivity analysis of OS.
The primary study endpoint was OS. Comparisons were performed using a log-rank test, with hazard ratios and 95% confidence intervals calculated using Cox models, stratified by extent of disease (nonregional lymph node versus bone +/- lymph node versus visceral ± lymph node/bone metastases) and alkaline phosphatase levels (< versus ≥ upper limit of normal). Patients with no documented death were censored at the last known alive or data cut-off date, whichever occurred earlier. The post hoc sensitivity analysis counted initiation of subsequent systemic antineoplastic therapy as an event in censored patients still alive at the end of follow-up. In addition, planned sensitivity analyses used an unstratified log-rank test/Cox model, a log-rank test/Cox model with stratification factors from electronic case report forms, and a log-rank test/Cox model with extent of disease stratification factors from central imaging review.
374 (76%) patients in the placebo arm received subsequent life-prolonging systemic therapy. Time to first subsequent systemic antineoplastic therapy, a key secondary endpoint, was significantly longer with triplet therapy (HR: 0.39, 95% CI: 0.33 to 0.46, p<0.001).

Results of the sensitivity analysis counting initiation of subsequent systemic antineoplastic therapy as an event in censored patients and the other planned sensitivity analyses demonstrated OS outcomes consistent with those observed in the primary analysis.

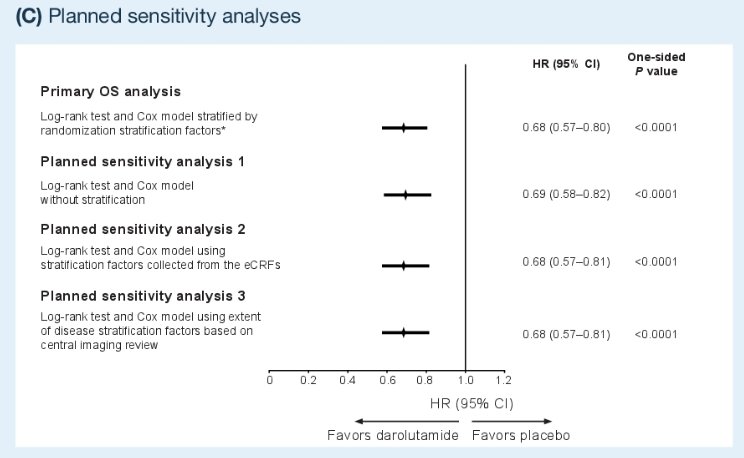
Dr. Shore concluded that the results of the post hoc and planned sensitivity analyses were consistent with and supportive of the ARASENS primary OS analysis. These data reinforce darolutamide + ADT + docetaxel as an effective and well-tolerated new standard of care for early treatment intensification in patients with mHSPC.
Presented by: Neal Shore, MD, FACS, Urologist, Director, CPI, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SCWritten by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related content: Triplet Delays Further Therapy Versus Doublet in Metastatic HSPC - Neal Shore
References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Jul 10;41(20):3595-3607.


