(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer poster session. Dr. Stefanie Zschaebitz presented the results of exploratory analyses of homologous recombination repair (HRR) gene subgroups and potential associations with secondary efficacy endpoints in the HRR-deficient population from TALAPRO-2.
TALAPRO-2 is a phase III randomized, double-blind, placebo-controlled trial that evaluated the combination of talazoparib and enzalutamide in the first-line treatment setting for mCRPC patients. Patients were randomized 1:1 to talazoparib 0.5 mg once daily (reduced dose from standard of 1.0 mg) plus enzalutamide 160 mg once daily versus placebo + enzalutamide. Prior use of docetaxel and abiraterone in the metastatic hormone sensitive (mHPSC), but not in the metastatic castrate-resistant prostate cancer (mCRPC) setting, was permitted. No prior use of an androgen receptor inhibitor was permitted. Notably, this was a biomarker unselected cohort of ‘all comers’. The primary endpoint was rPFS, assessed via blinded independent central review (BICR), and overall survival (OS) was a key secondary endpoint. The trial design for TALAPRO-2 is as follows:
21% of patients in each arm were HRR deficient. The combination of talazoparib + enzalutamide was associated with a 37% improvement in rPFS, compared to placebo + enzalutamide (median: not reached versus 22 months; HR: 0.63, 95% CI: 0.51 – 0.78, p<0.001). OS data remains immature, with a trend towards an OS improvement in the overall cohort (HR: 0.89, 95% CI: 0.69 – 1.14, p=0.35).1
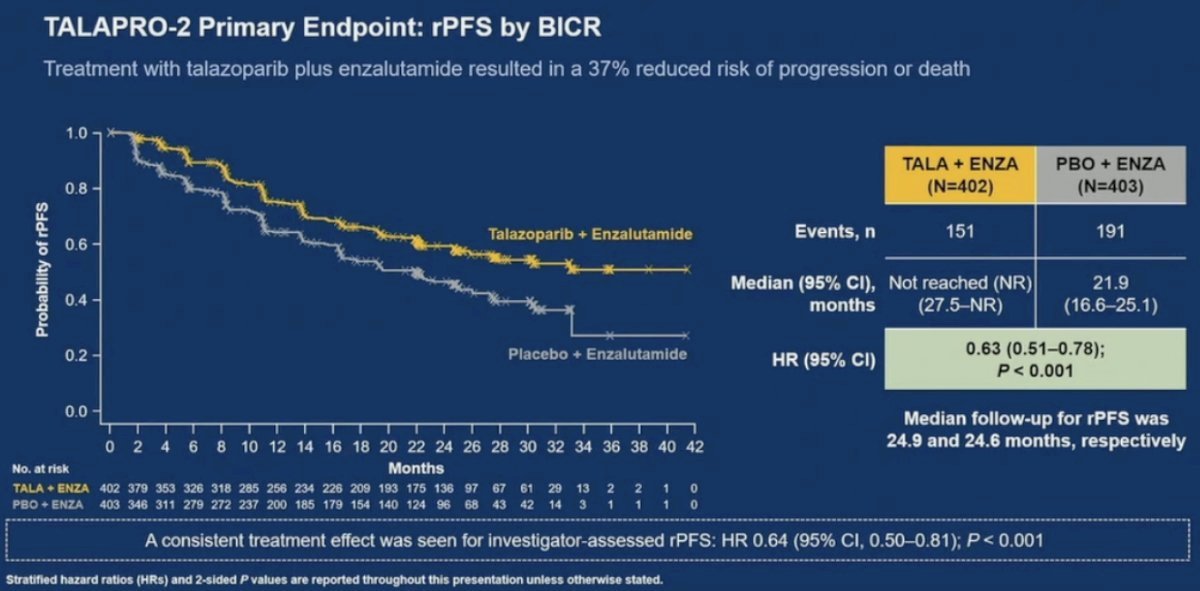
In this report, Dr. Zschaebitz and colleagues reported exploratory biomarker analyses assessing HRR gene subgroups and potential associations with secondary efficacy endpoints in patients with HRRm tumors. Prospective gene testing used a 12-gene HRR panel (HRR12) of FoundationOne CDx/FoundationOne Liquid CDx. For single gene groups, only patients bearing alteration(s) in that gene and no other HRR12 genes were analyzed. The gene cluster alteration dominance hierarchy was as follows:
- Any BRCA1/2 (BRCA cluster)
- Any PALB2 (PALB2 cluster)
- Any CDK12 (CDK12 cluster)
- Any ATM (ATM cluster)
- All other HRR12 genes (each patient counted once)
The evaluated study endpoints included:
- Objective response rate (ORR)
- Time to progression or death on first subsequent antineoplastic therapy (PFS2)
- PSA response ≥50% (PSA50)
- Time to PSA progression
- Time to initiation of cytotoxic chemotherapy
The addition of talazoparib to enzalutamide demonstrated a differential benefit for the BRCA2 single gene group across all endpoints:
- ORR: 79% versus 33% (OR: 0.13, 95% CI: 0.03 – 0.63)
- PFS2: Median not reached versus 26 months (HR: 0.44, 95% CI: 0.20 – 0.97)
- PSA50: 87% versus 58%
- Time to PSA progression: Median not reached versus 9.2 months (HR: 0.20, 95% CI: 0.10 – 0.41)
- Time to initiation of cytotoxic chemotherapy: Median not reached versus 17 months (HR: 0.27, 95% CI: 0.13 – 0.57)
A similar broad differential benefit was evidence for the CDK12 single gene group:
- ORR: 70% versus 29% (OR: 0.17, 95% CI: 0.01 – 2.03)
- PFS2: 36.4 versus 19 months (HR: 0.29, 95% CI: 0.11 – 0.75)
- PSA50: 86% versus 53%
- Time to PSA progression: 14 versus 11 months (HR: 0.61, 95% CI: 0.29 – 1.26)
- No benefit was observed for time to initiation of cytotoxic chemotherapy (HR: 1.32, 95% CI: 0.43–4.06)
For ATM single gene group, a differential numerical benefit was seen for:
- ORR: 82% versus 20% (OR: 0.06, 95% CI: 0 – 1.17)
- Time to PSA progression: 27 versus 16 months (HR: 0.61, 95% CI: 0.25 – 1.47)
- Time to initiation of cytotoxic chemotherapy (HR 0.60, 95% CI: 0.18 – 1.96)
- No differential benefit was seen in other endpoints
The CHEK2 single gene group showed a numerically differential benefit for PSA endpoints and time to initiation of cytotoxic chemotherapy. The other HRR12 gene cluster overall showed comparable efficacy between arms with potential exception of time to PSA progression (median: 28.6 versus 14 months; HR 0.65, 95% CI: 0.33–1.31), and time to initiation of cytotoxic chemotherapy (median: not reached for both arms, HR: 0.65, 95% CI: 0.26–1.62).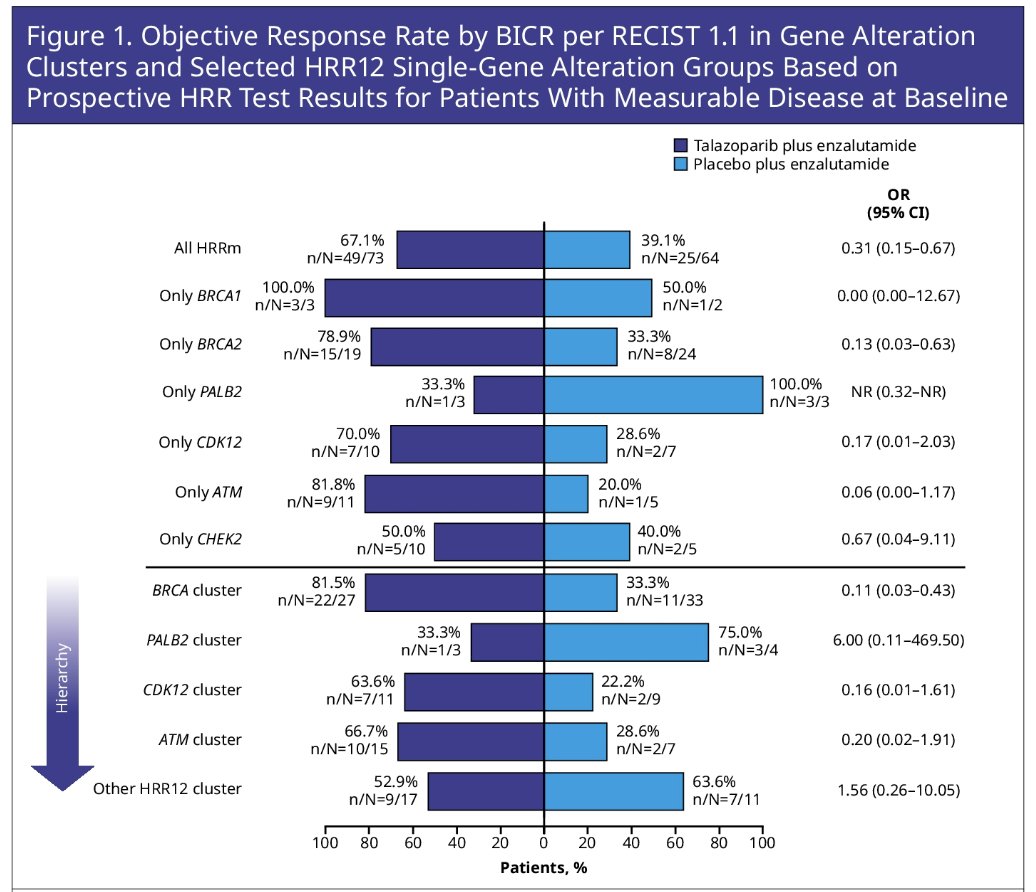
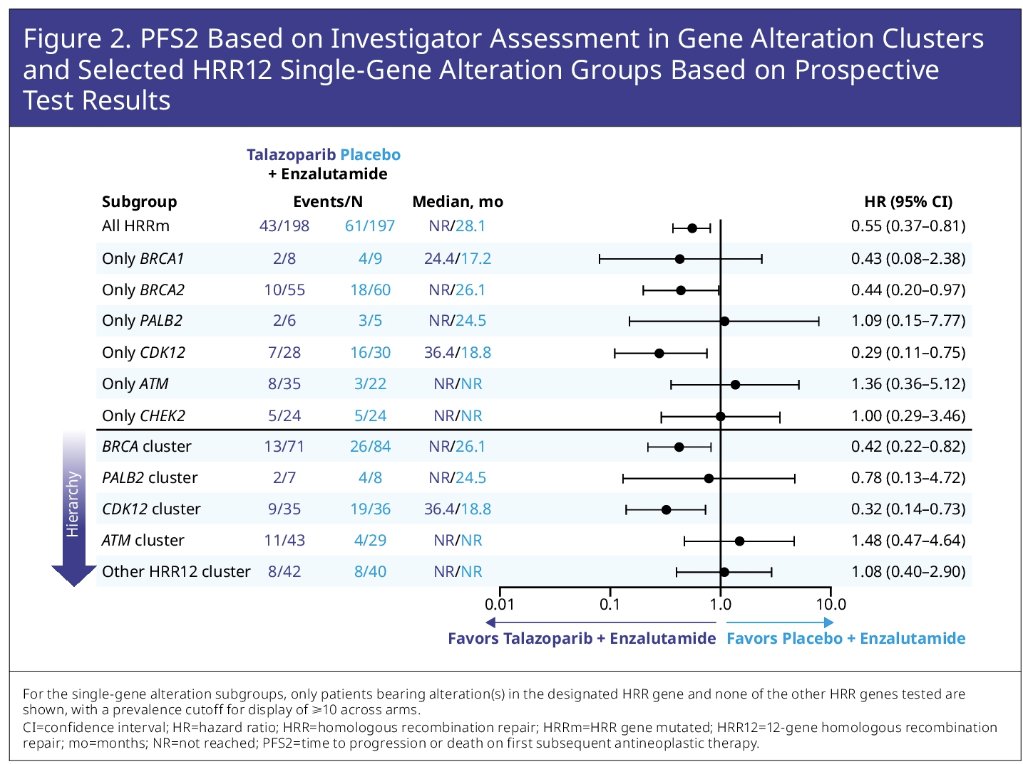
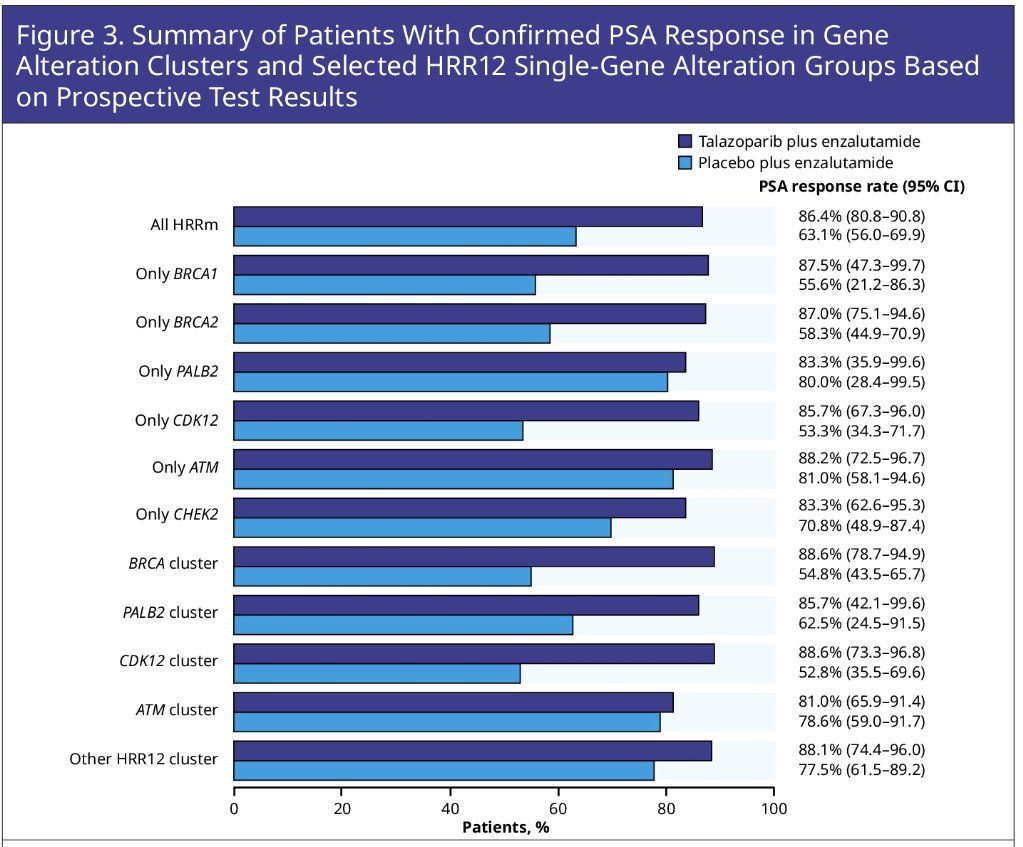
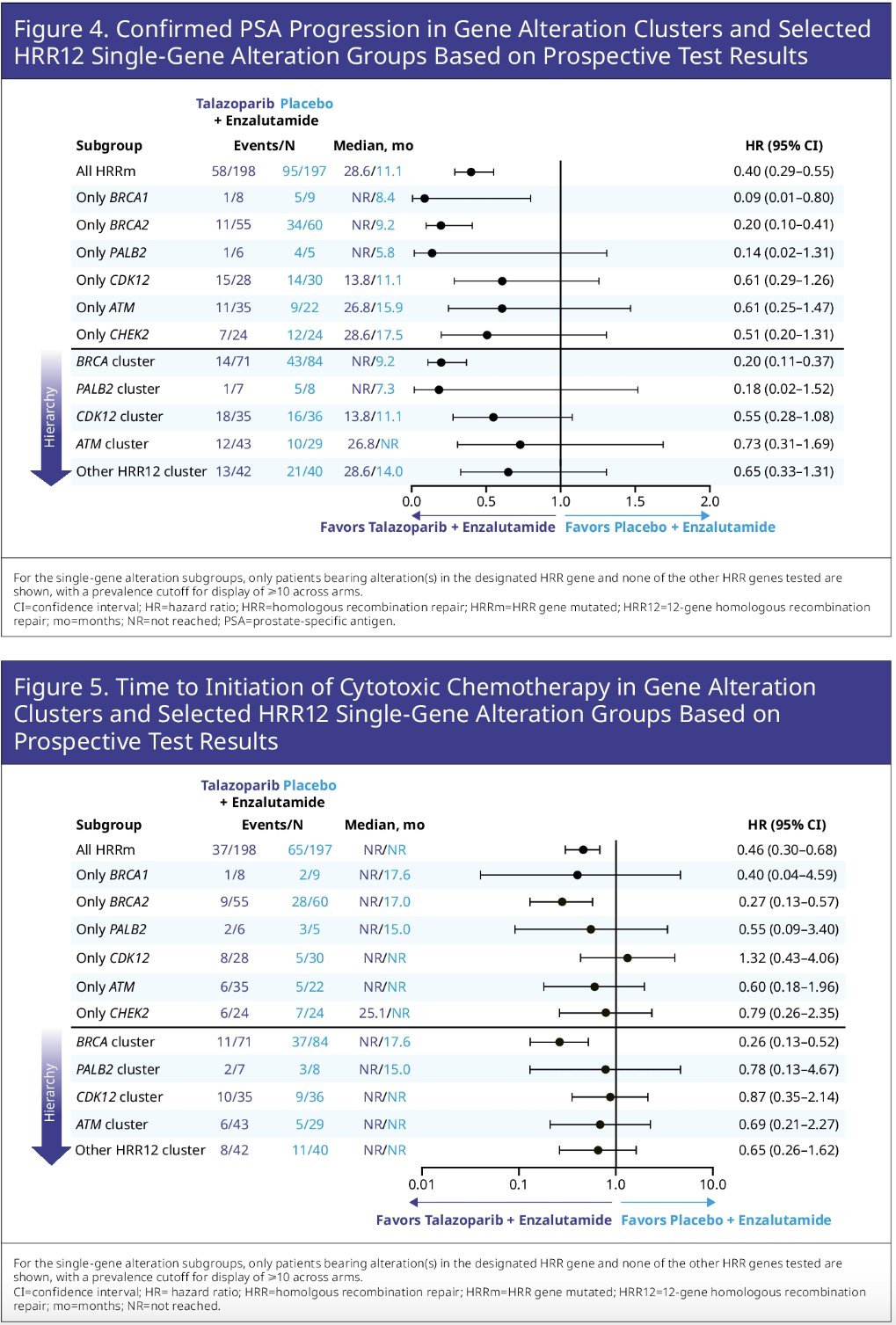
Dr. Zschaebitz concluded that a broad differential efficacy benefit was evident for talazoparib + enzalutamide versus placebo + enzalutamide across multiple molecular subgroups and was most pronounced for the BRCA1-PALB2-BRCA2 axis and CDK12. Additional analyses are warranted.
Presented by: Stefanie Zschaebitz, MD, Department of Medical Oncology, National Center for Tumor Diseases, Heidelberg University Hospital, Heidelberg, Germany
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:

