(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer poster session. Dr. Neal Shore presented the result of a real-world analysis of homologous recombination repair (HRR) gene mutation testing patterns and treatment selection from a real-world cohort of patients with metastatic castration-resistant prostate cancer (mCRPC).
Routine germline and somatic testing of mCRPC patients in clinical practice is recommended by international guidelines for all mCRPC patients, particularly given the approval of numerous poly ADP ribose polymerase (PARP) inhibitors for the biomarker-selected treatment of patients with specific HRR gene mutations, both in combination with androgen receptor pathway inhibitors in the 1st line setting (PROpel,1 TALAPRO-2,2 MAGNTIUDE3), and as single agents in later-line settings (e.g., PROfound4 and TRITON25). Despite these regulatory approvals and guidelines recommendations, analyses suggest that many mCRPC patients are not offered testing, which limits the arsenal of potential treatment options for HRR mutation-positive patients, particularly with regard to potential PARP inhibitor therapy. In this report, Dr. Shore and colleagues reviewed HRR mutation testing and treatment patterns within a real-world cohort of mCRPC patients, in order to identify potential clinical practice gaps.
A de-identified real-world cohort of newly diagnosed and actively treated mCRPC patients, excluding those with lymph node-only metastasis, were identified between January 2020 and December 2021 from the IntegraConnect – Precision Q Database that includes electronic, practice management and payer data from 500 US sites of care. Patient charts were manually reviewed by medical curators to extract data including:
- Frequency and timing of germline and somatic testing for HRR mutations
- Testing modality (tissue or liquid biopsy)
- Results of HRR mutation testing
- Use of PARP inhibitors in the HRR mutation positive patient cohort.
This analysis included a total of 996 mCRPC patients. 93% were managed by community oncologists, 6% by community urologists, and 1% by academic oncologists. Almost 60% of patients received testing for HRR mutations (germline: 20%, somatic: 60%, germline + somatic: 4%, unknown: 16%).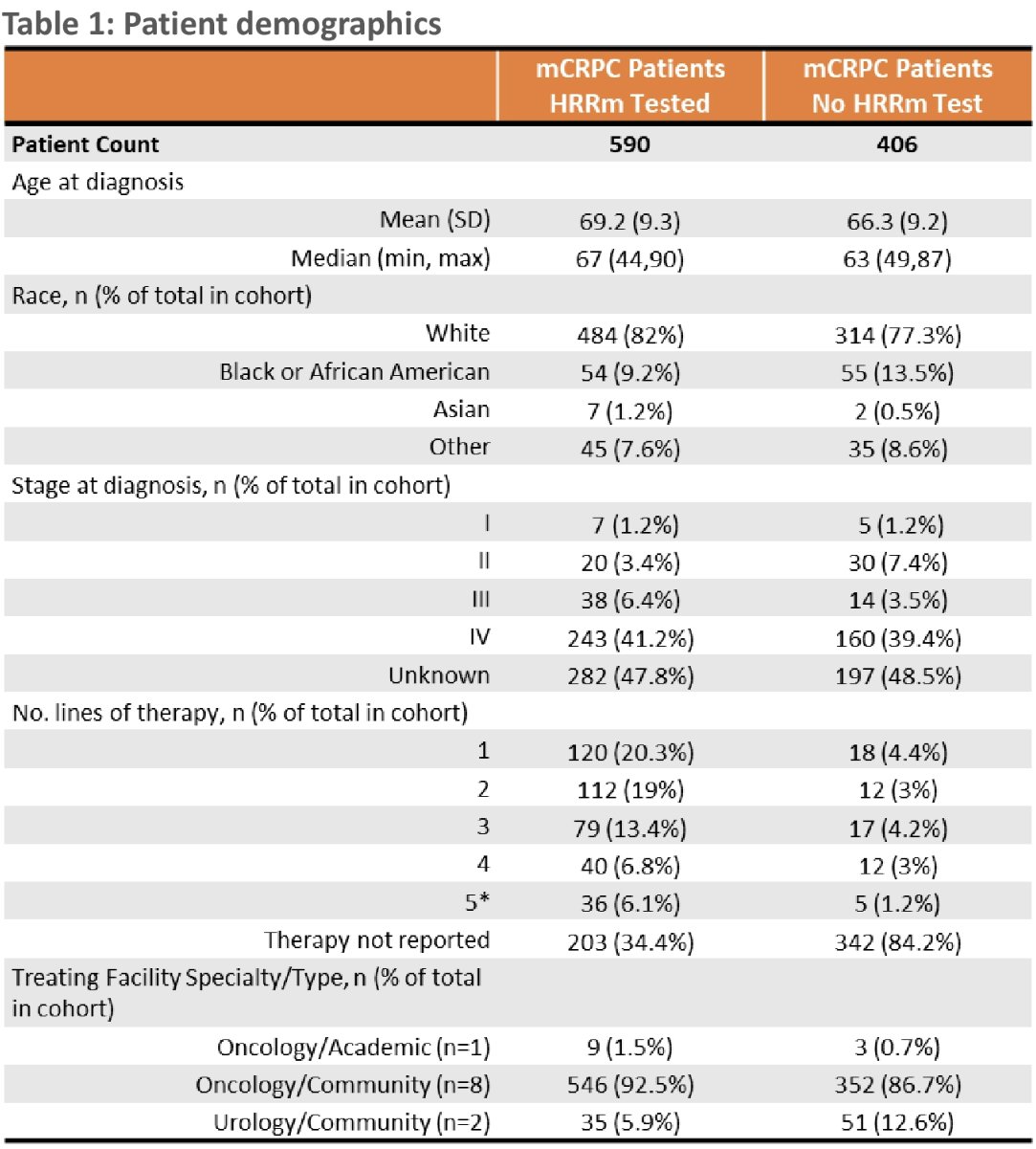
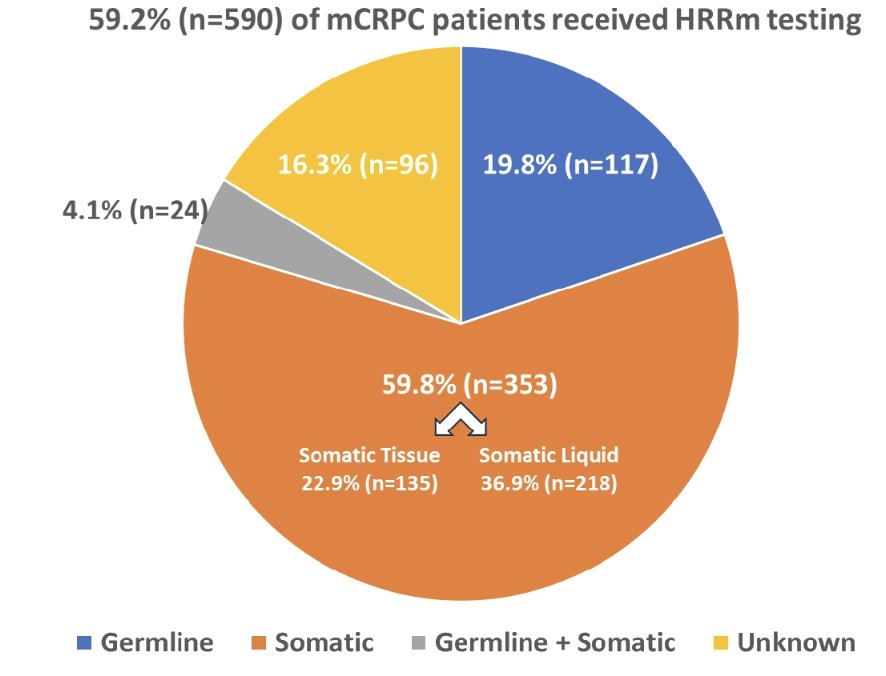
Testing rates increased following PARP inhibitor approval:
- January – May 2020: 15%
- June – September 2020: 23%
- October 2020 – December 2021: 62%

The vast majority (64%) were tested after receiving mCRPC treatment, with 48% tested after failure on first line therapy.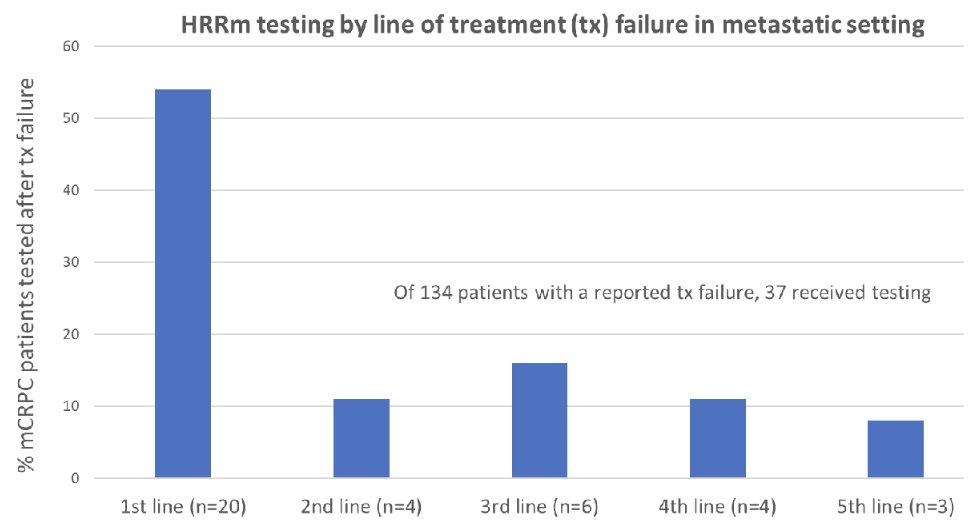
Somatic tissue testing was the predominant testing modality, compared to liquid biopsy and germline testing. 32% of tested patients were found to be HRR mutation positive (germline: 11%, somatic: 74%, germline + somatic: 2%, unknown: 13%), although not all tests utilized included the 14 HRR genes in the olaparib approval. BRCA2, ATM, CHEK2, and CDK12 HRR mutations were the most commonly detected, with a higher frequency of ATM and CHEK2 mutations seen in liquid biopsies. Of the mCRPC patients who were HRR mutation positive, 67% received a PARP inhibitor.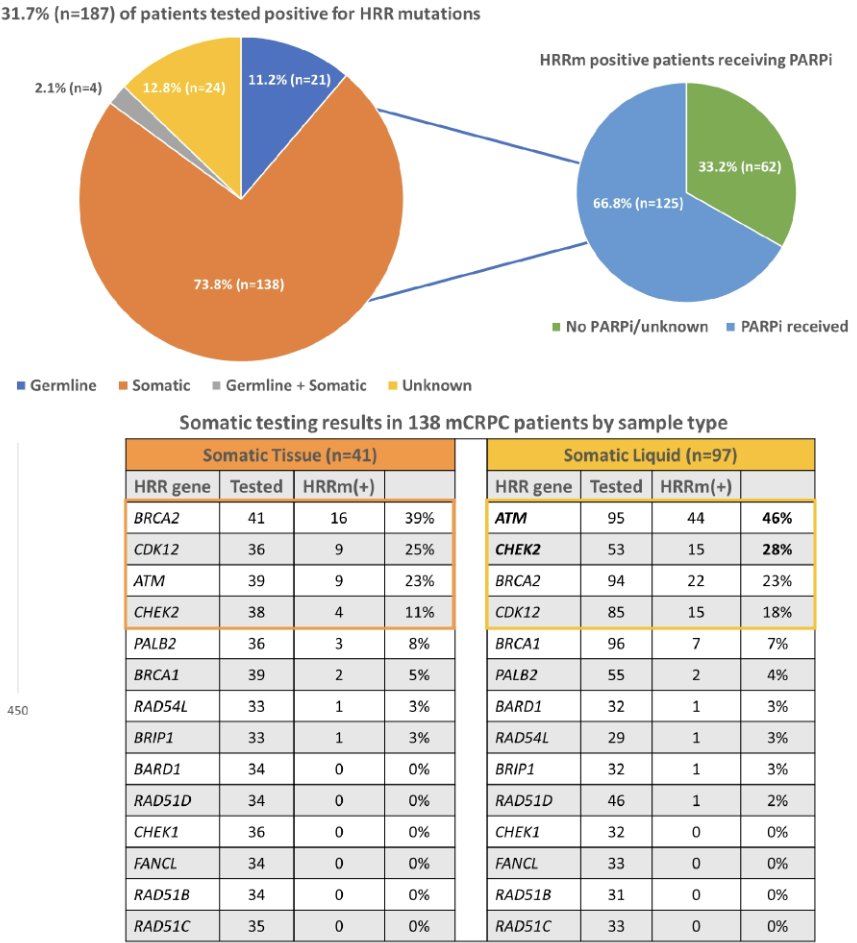
Dr. Shore concluded that in this real-world analysis, 40.8% of mCRPC patients did not receive germline or somatic testing, while 33.2% of HRR mutation positive patients did not receive PARP inhibitors. Optimizing germline and somatic testing for mCRPC is a significant unmet need, which negatively impacts therapeutic offerings.
Presented by: Neal Shore, MD, FACS, Urologist, Director, CPI, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Nov 10;38(32):3763-3772.


