(UroToday.com) The 2024 GU ASCO annual meeting featured an oral prostate cancer session and a presentation by Dr. Neeraj Agarwal discussing CONTACT-02, a phase 3 study of cabozantinib + atezolizumab vs second novel hormonal therapy in patients with metastatic castration-resistant prostate cancer (mCRPC). Patients who have progressed on a prior novel hormonal therapy and have mCRPC with extrapelvic nodal or visceral metastasis have a poor prognosis with limited treatment options. After a novel hormonal therapy, chemotherapy or a second novel hormonal therapy are the only broadly available non-targeted treatments, with limited chemotherapy use secondary to toxicity and frailty from previous treatment.
Cabozantinib is a multi-targeted TKI that showed an rPFS and overall survival (OS) benefit in a subgroup of patients with visceral metastasis in the phase 3 COMET-1 study of patients with treatment refractory mCRPC. Cabozantinib also promotes an immune-permissive tumor environment and may enhance response to immune checkpoint inhibitors. The phase 3 CONTACT-02 trial evaluated the use of cabozantinib + atezolizumab versus a second novel hormonal therapy in mCRPC with measurable extrapelvic soft tissue metastasis (visceral or lymph nodes) after progression on a first novel hormonal therapy.
Patients were randomized 1:1 to cabozantinib + atezolizumab (cabozantinib [40 mg PO daily] + atezolizumab [1200 mg IV every 3 weeks]) or control (abiraterone [1000 mg PO daily] + prednisone [5 mg PO twice daily] or enzalutamide [160 mg PO daily]) and were stratified by liver metastasis (yes/no), prior docetaxel for mCSPC (yes/no), and prior novel hormonal therapy for mCSPC, M0CRPC, or mCRPC. The trial design for CONTACT-02 is as follows: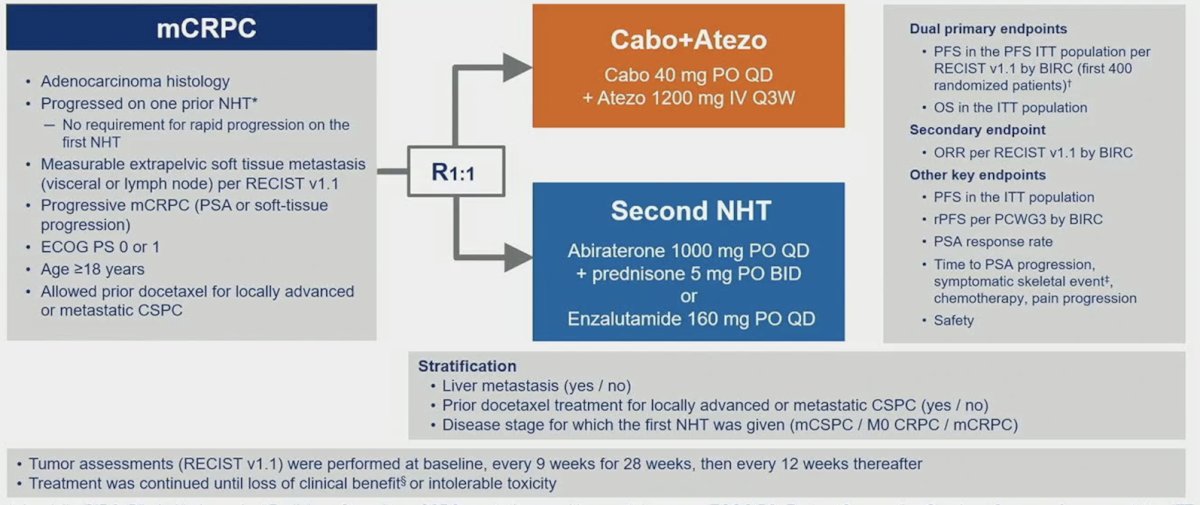
Key eligibility criteria included (i) mCRPC with disease progression on one prior novel hormonal therapy, (ii) measurable extrapelvic nodal or visceral disease, (iii) ECOG PS ≤1, and (iv) ongoing ADT. Of note, docetaxel was allowed for mCSPC. Dual primary endpoints were radiographic PFS by a blinded independent radiology committee (BIRC) per RECIST 1.1 in the first 400 randomized patients and overall survival in all randomized patients. A key secondary endpoint was the objective response rate by RECIST 1.1 per BIRC. From a statistical standpoint, PFS was analyzed after 202 events in the PFS ITT population (first 400 patients), with an alpha of 0.002 and power of 90%. The final OS will be analyzed after ~340 deaths in the ITT population with an alpha of 0.048 and power of 90%, or an alpha of 0.05 if PFS is positive (fallback method). Objective response rate is tested only if the null hypothesis for OS is rejected in favor of the experimental group.
At the data cutoff (February 28, 2023), 507 patients were randomized to receive cabozantinib + atezolizumab (n = 253) or to the control group (n = 254). The patient disposition is as follows: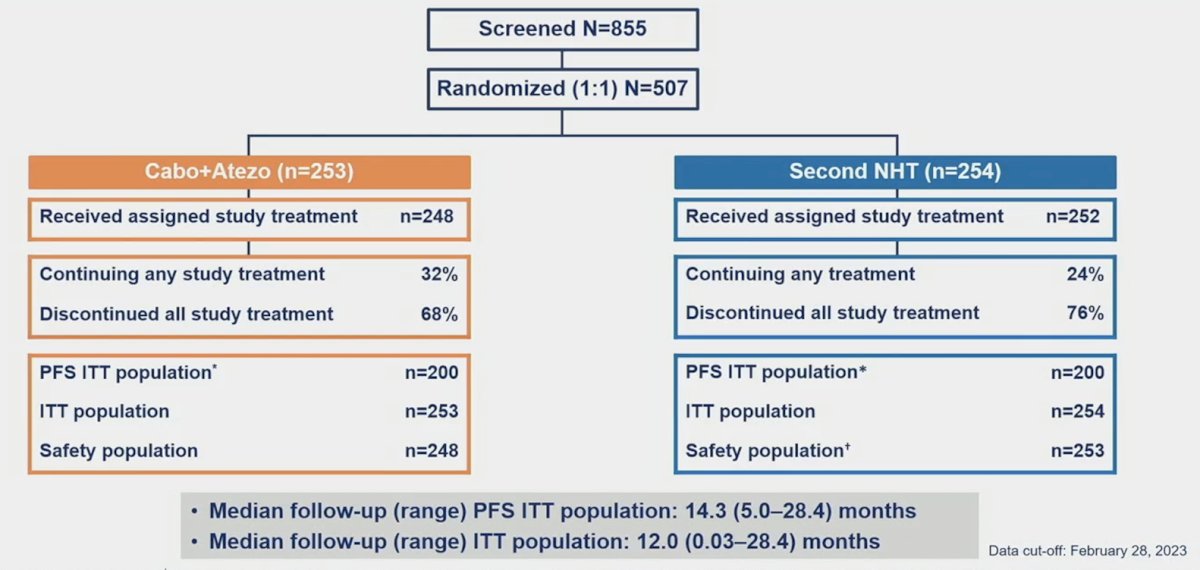
Baseline and clinical characteristics were balanced between cabozantinib + atezolizumab and control arms: 23% and 24% had liver metastasis, 23%, and 23% received docetaxel for mCSPC, and 72% and 74% received first novel hormonal therapy for mCRPC: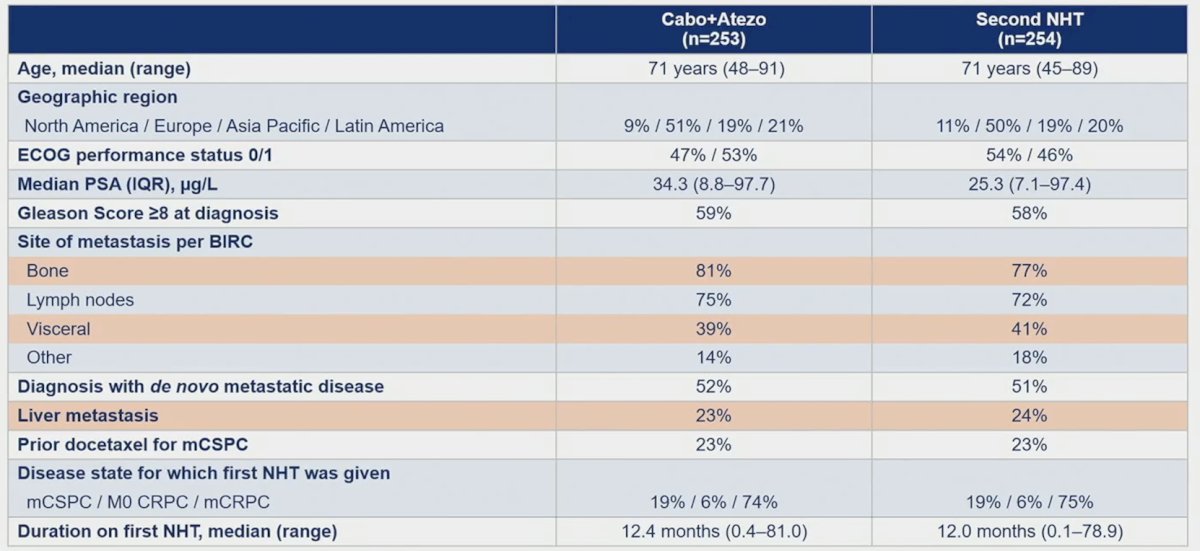
The median follow-up was 12.0 months for all randomized patients and 14.3 months for the first 400 patients. The median radiographic PFS was significantly longer with cabozantinib + atezolizumab vs control (6.3 vs 4.2 months; HR 0.65, 95% CI 0.50-0.84):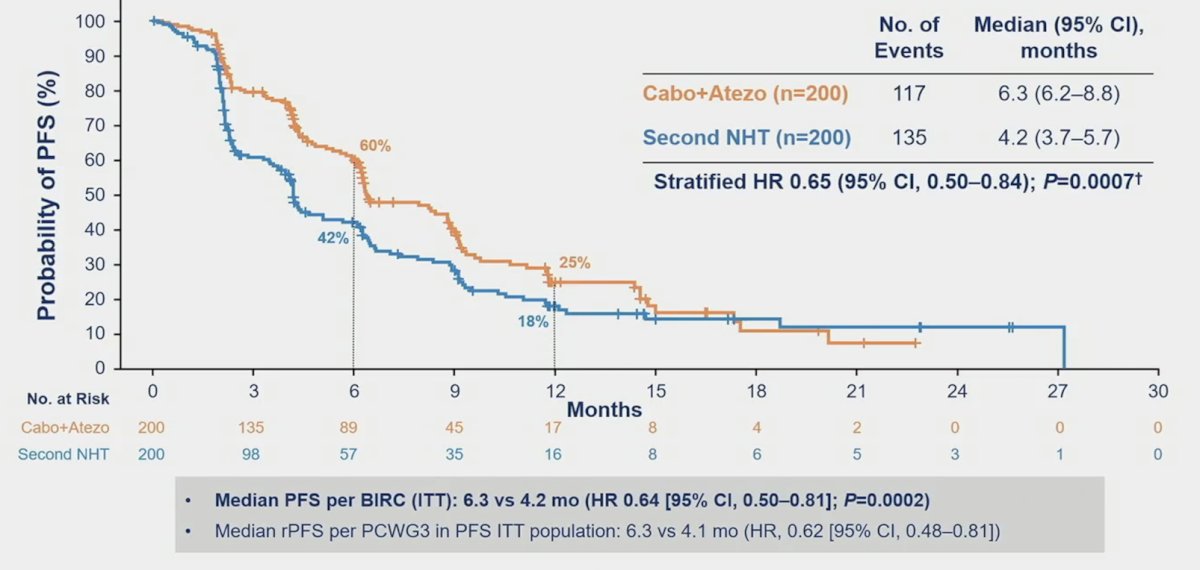
PFS was also improve in prespecified subgroups with liver metastasis (6.0 vs 2.1 months; HR 0.47, 95% CI 0.30- 0.74), prior docetaxel treatment for mCSPC (8.8 vs 4.1 months; HR 0.55, 95% CI 0.32-0.96), and bone metastasis (6.3 vs 4.1 months; HR 0.67, 95% CI 0.50-0.88):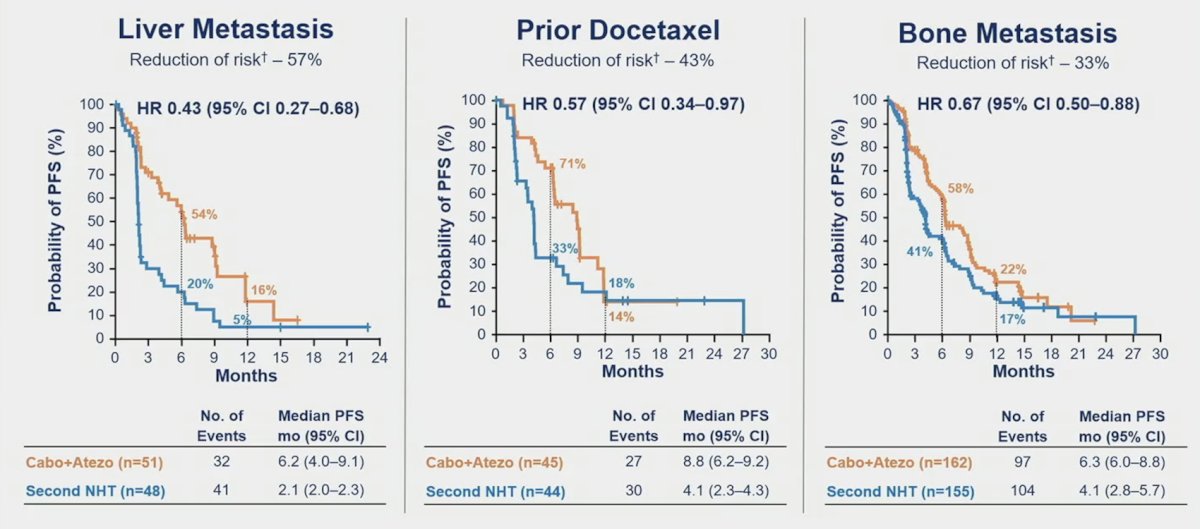
The interim OS analysis in the ITT population (49% of target events) showed a trend towards improvement with cabozantinib + atezolizumab (HR 0.79, 95% CI 0.58-1.07):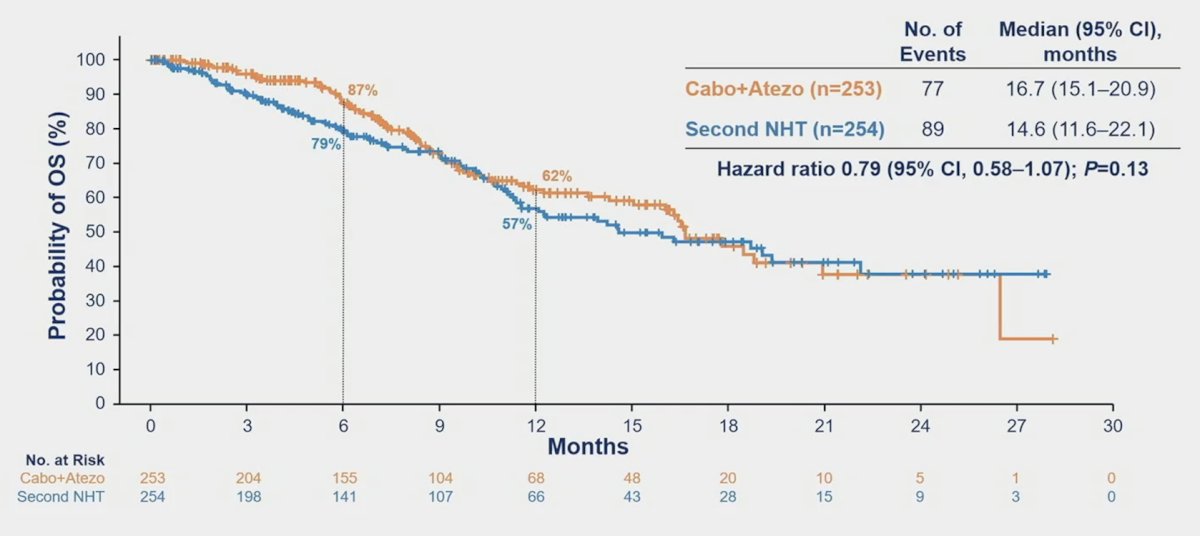
The objective response rate was higher in cabozantinib + atezolizumab vs control in patients with follow-up ≥6 months in all randomized patients (14% vs 4%):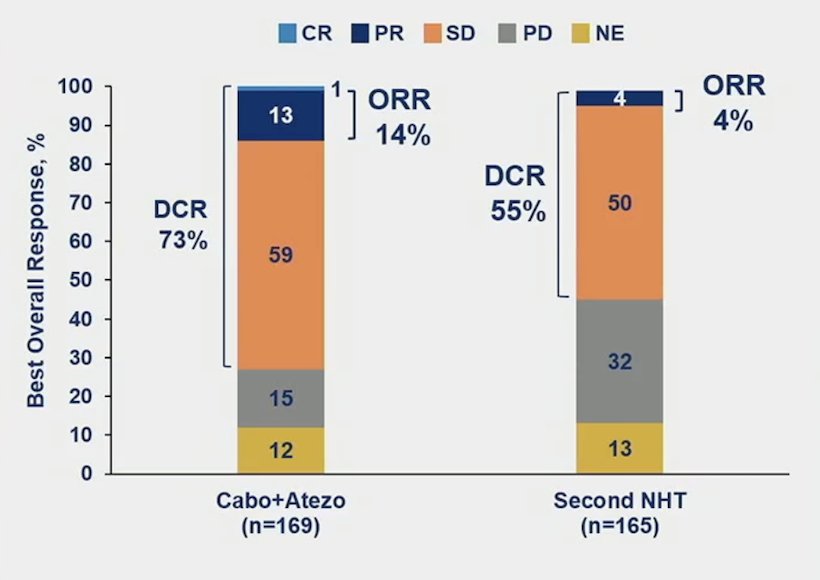
The median duration of response was 9.7 months for cabozantinib + atezolizumab versus not reached for the control arm, and time to response was 2.3 vs 4.6 months, respectively. Disease control rate was 72.8% (123/169) vs 54.5% (90/165), respectively. Other key endpoints, including median time to chemotherapy favoring cabozantinib + atezolizumab (HR 0.56, 95% 0.39-0.82) are as follows: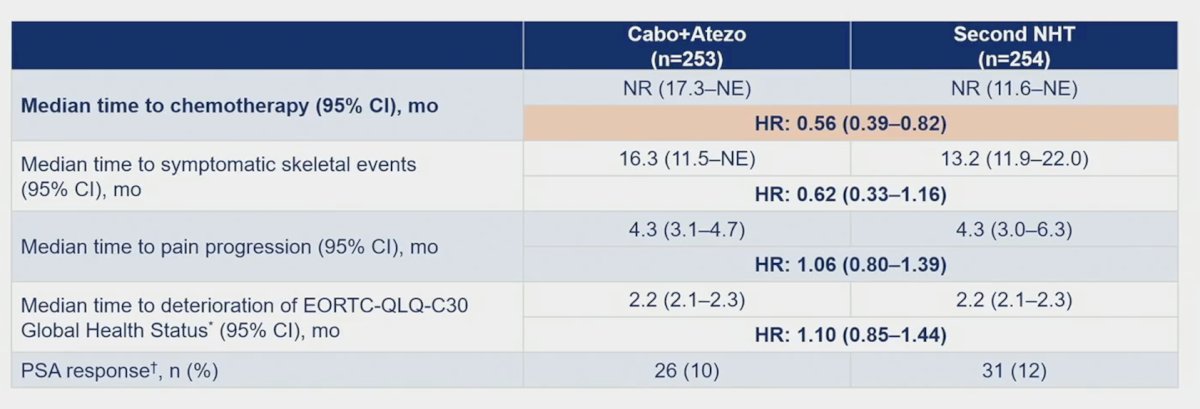
Treatment-emergent adverse events occurred in 97% in cabozantinib + atezolizumab vs 87% in the control arm (grade 3/4 events, 48% vs 23%). Grade 5 treatment-emergent adverse events occurred in 9% vs 12% and no grade 5 treatment-related adverse events occurred in either arm: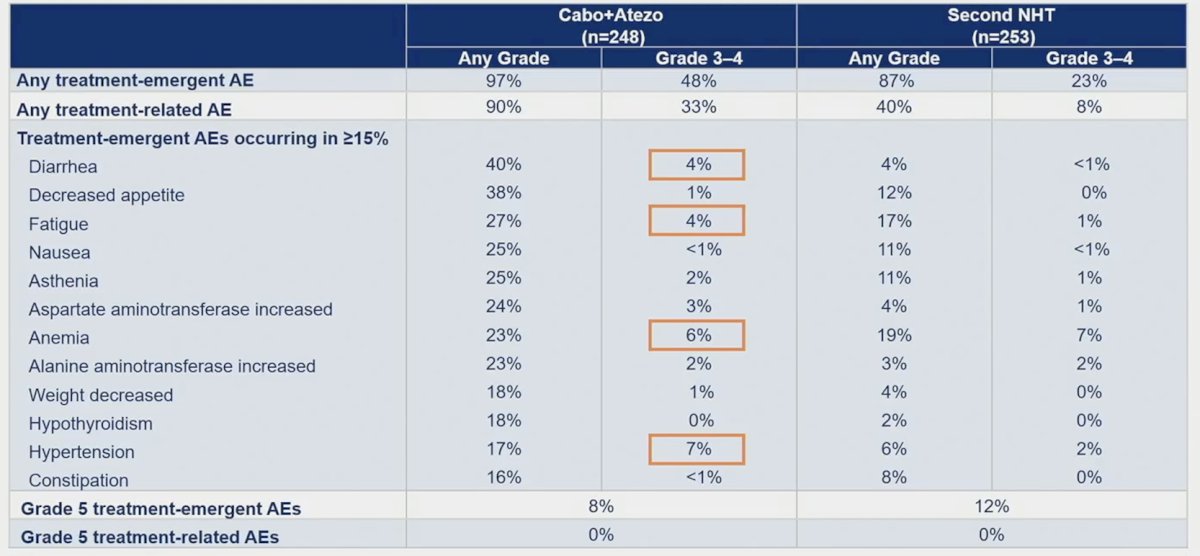
Treatment-emergent adverse events led to the discontinuation of all treatment components in 16% in cabozantinib + atezolizumab and 15% in the control arm.
Dr. Agarwal concluded his presentation discussing the randomized phase 3 CONTACT-02 trial with the following take-home points:
- Cabozantinib + atezolizumab significantly improved radiographic PFS vs second novel hormonal therapy in patients who had progressed on a prior novel hormonal therapy and have mCRPC with extrapelvic nodal or visceral disease, a population with a high unmet medical need
- These radiographic PFS benefits were particularly notable in patients with liver metastasis and those who previously received docetaxel for mCSPC
- OS data is immature, but there was a trend toward improvement with cabozantinib + atezolizumab, including high-risk populations
- Toxicities reported with each treatment arm were manageable
- CONTACT-02 is the only phase 3 study of an immune checkpoint inhibitor-based regimen to show a significant and clinically meaningful improvement in radiographic PFS in prostate cancer with visceral metastasis
- Follow-up for overall survival is ongoing
- These data support the combination of cabozantinib + atezolizumab as a potential new treatment options for patients with mCRPC who have progressed with a novel hormonal therapy
Presented by: Neeraj Agarwal, MD, Huntsman Cancer Institute at the University of Utah, Salt Lake City, UT
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
Related Content: Exelixis Announces Detailed Results of Phase 3 CONTACT-02 Pivotal Trial Evaluating Cabozantinib in Combination with Atezolizumab in Metastatic Castration-Resistant Prostate Cancer Presented at ASCO GU 2024
CONTACT-02 Phase III Trial Findings on Cabozantinib-Atezolizumab Combination in mCRPC - Neeraj Agarwal


