(UroToday.com) The 2024 GU ASCO annual meeting featured an oral prostate cancer session and a presentation by Dr. Maha Hussain discussing BRCAAway, a randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) bearing homologous recombination-repair (HRR) mutations. The PARP inhibitor olaparib is approved for mCRPC patients with deleterious germline or somatic HRR mutations, which is notable given that deleterious germline or somatic HRR mutations are present in about 20% of mCRPC patients. Pre-clinically, PARP-inhibition has demonstrated synergism with AR-targeted therapy. The BRCAAway trial is a biomarker pre-selected, multicenter, randomized, phase-2 trial which evaluated efficacy of AR-inhibitor vs PARP inhibitor vs combination in first-line mCRPC patients with germline and/or somatic mutations in BRCA1/2 or ATM. The hypothesis is that co-targeting the androgen receptor and PARP1 in mCRPC patients with HRR mutations will result in higher and more durable responses.
Eligibility for BRCAAway required front-line mCRPC with no prior exposure to PARP inhibitor, AR-inhibitor, or chemotherapy for mCRPC, and washout period for an antiandrogen, radiation, and other investigational agents. Eligible patients underwent tumor next-generation sequencing/germline testing, and patients with inactivating BRCA1/2 and/or ATM alterations were randomized 1:1:1 to:
- Arm I abiraterone (1000 mg daily) + prednisone (5mg twice daily)
- Arm II olaparib (300 mg twice daily)
- Arm III olaparib + abiraterone/prednisone
The primary endpoint was progression free survival as per RECIST 1.1, PCWG3, clinical assessment, or death. The secondary endpoints included measurable disease response rate, PSA response rate, and toxicity. Arm I and II patients were permitted to cross over at the time of progression. The trial design for BRCAAway is as follows:
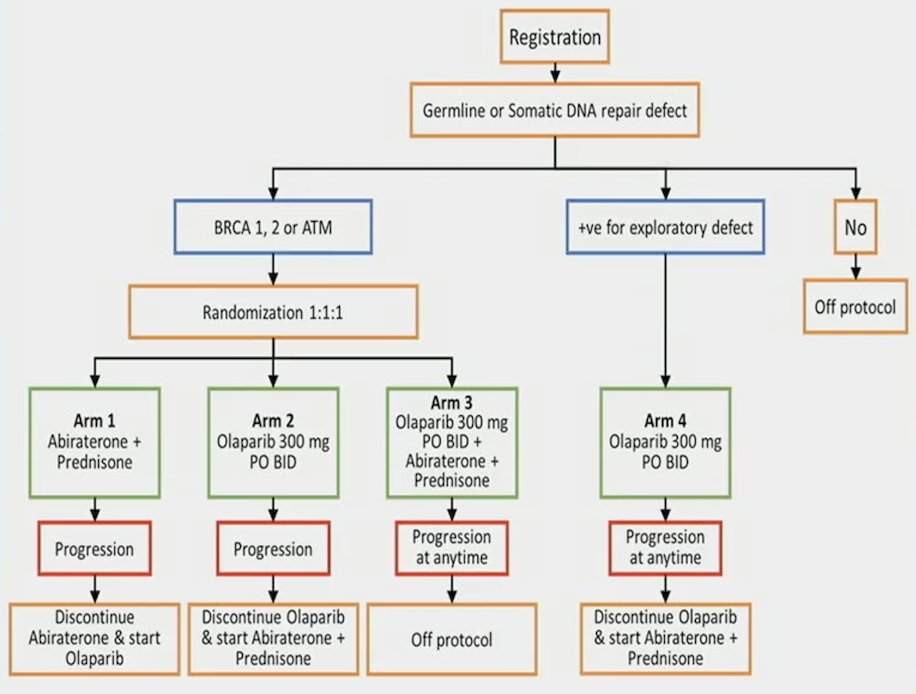
The statistical design included a Simon’s randomized phase II selection design with 20 patients per arm. With 60% 12-month PFS in this population versus 40% in historic controls, there would be 83% probability of selecting the best treatment among Arms I-III.
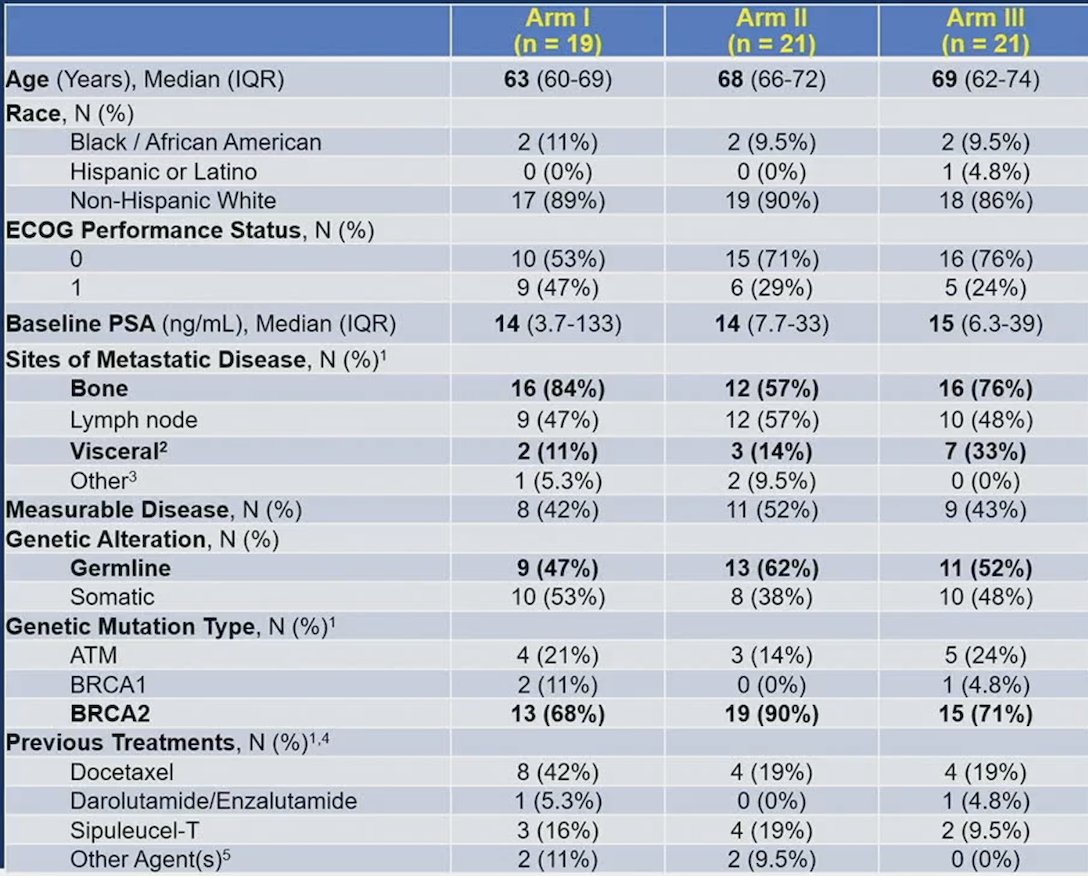
There were 165 eligible patients registered that underwent next-generation sequencing/germline testing, of which 61 patients with HRR mutations were randomized to Arms I-III. The median age was 67 years (range 42-85), 55 patients were white, 6 patients were black, 26% of patients had prior therapy with docetaxel for mHSPC, and 3.3% of patients had prior darolutamide/enzalutamide for nmCRPC. The disease sites affected include 44 patients with bone involvement, 12 with visceral disease, 31 with lymph node positivity, and 3 with other sites. The median baseline PSA was 14 ng/ml (range 0.15-4,037 ng/ml). With regards to HRR mutations status, 3 patients had a BRCA1 mutation, 46 a BRCA2 mutation, and 11 patients had an ATM mutation, as well as one patient having multiple mutations (33 germline, 28 somatic). The baseline characteristics stratified by treatment arms is as follows:
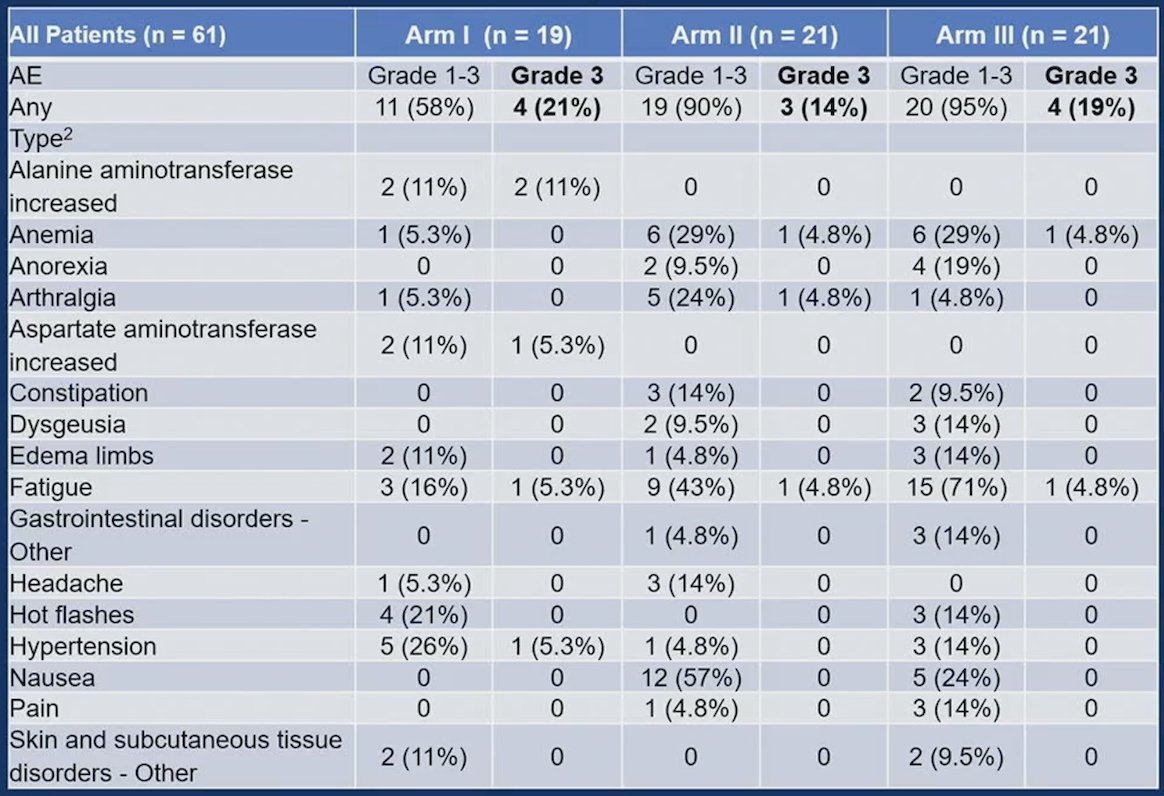
The median time from randomization to last encounter in patients still alive (n = 56) was 16 months (range: 0.8-60) for Arm I, 15 months (range: 4.1-36) for Arm II, and 23 months (range: 2.9-56) for Arms III. There were 51 patients with treatment-related adverse events, with the most common Grade 3 adverse events being fatigue, anemia, and ALT increases:
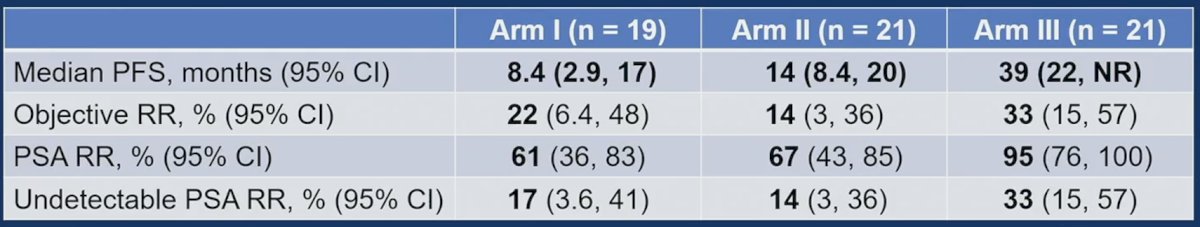
Overall survival data is not mature, with 3 deaths in Arm I and 2 in Arm II. Efficacy results for Arms I-III are presented in the table, with notable improvements in PFS, objective response rate, PSA response rate, and undetectable PSA response rate in Arm III compared to Arms I and II:
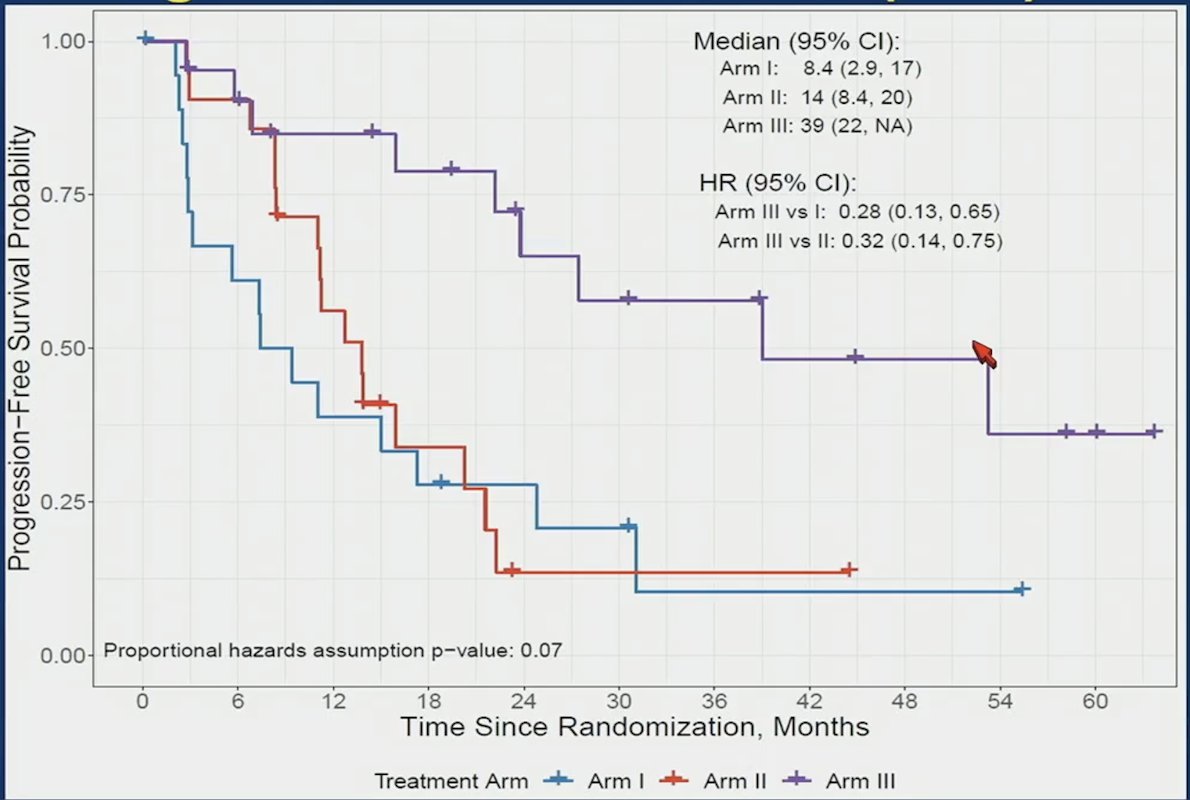
Progression free survival, stratified by treatment arm shows a significant benefit for Arm III vs I (HR 0.28, 95% CI 0.13-0.67) and Arm III vs II (HR 0.32, 95% CI 0.14-0.75):
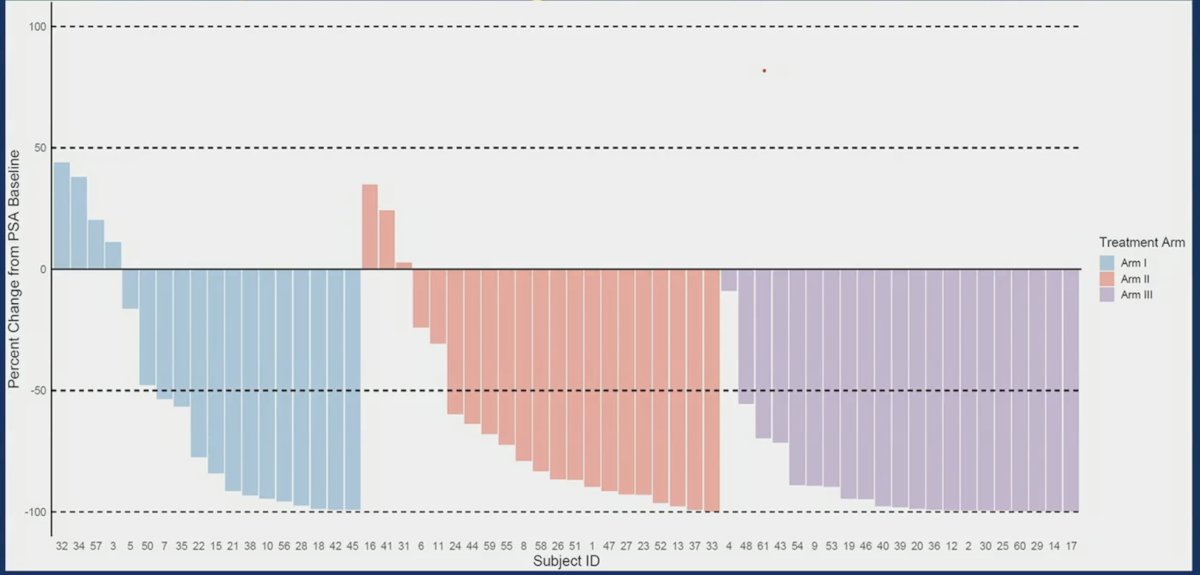
The PSA response (percent change from baseline to lowest PSA) per treatment arm shows impressive results for Arm III:
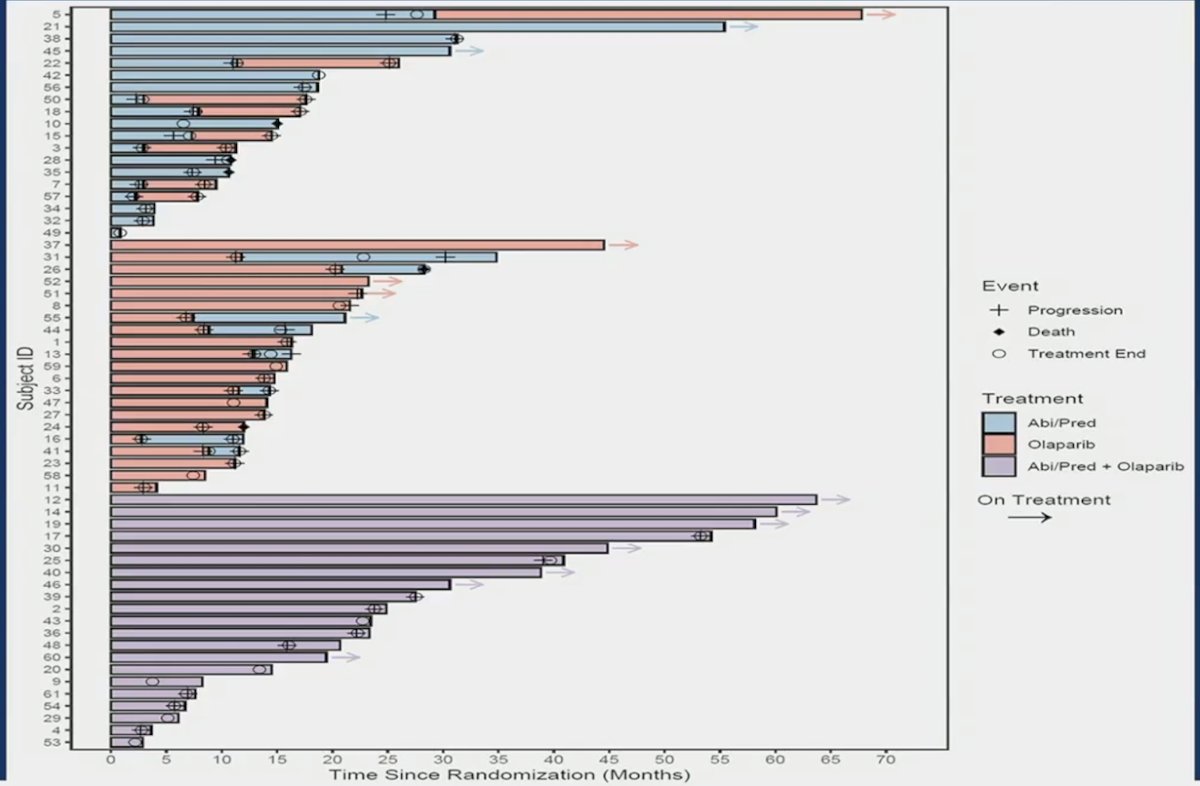
At progression, 8 of 19 patients crossed over from abiraterone to olaparib and 8 of 21 patients from olaparib to abiraterone. The median progression free survival from crossover to olaparib was 8.3 months (95% CI 5.5, 15) and to abiraterone was 7.2 months (95% CI 2.8, NR). The median progression free survival from randomization to olaparib was 16 months (95% CI 7.8-25) and to abiraterone was 16 months (95% CI 11-28). Response rate to crossover treatment was 38% for olaparib 38% and 25% for abiraterone. PSA response rate to crossover treatment was 50% for olaparib and 63% for abiraterone. The PFS swimmer plots, including crossover is highlighted below:
Dr. Hussain concluded her presentation discussing the randomized phase 2 BRCAAway trial with the following take-home points:
- In mCRPC patients with BRCA1/2 or ATM alterations, abiraterone/prednisone + olaparib was well tolerated and resulted in a longer progression free survival vs either agent alone or sequentially
- While the number of patients who crossed over was small, the front line combination had better PFS compared to sequential therapy
- On May 31, 2023, the FDA approved the combination of olaparib + abiraterone/prednisone as front line therapy for patients with mCRPC and BRCA1/2 alterations
Presented by: Maha H. A. Hussain, MD, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related Content:
BRCAAway - a Randomized Phase 2 Trial of Abiraterone, Olaparib, or Abiraterone + Olaparib in Patients with mCRPC with DNA Repair Defects - Maha Hussain
BRCAAWAY Trial: Impact of Abiraterone and Olaparib Combination in Prostate Cancer - Maha Hussain
BRCAAway Trial: Where Combination Treatment Improve Patient Outcomes in mCRPC - Daniel George


