(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer oral session discussing treatment intensification options for patients with early castration-resistant prostate cancer (CRPC) who had previously received doublet or triplet therapy. In her presentation, Dr. Valerie Fonteyne discussed the potential role(s) for stereotactic body radiotherapy (SBRT) for select patients with mCRPC.
Going back to the earlier case presentation at the beginning of this session, the case example was that of a de novo, high-volume metastatic hormone-sensitive prostate cancer patient who received triplet therapy. Eighteen months following the completion of his chemotherapy, his PSA increased to 1.25 ng/ml, and he began experiencing pelvic pain, which Dr. Fonteyne highlighted was of utmost clinical significance, as she would later elaborate on. A PSMA PET showed the same distribution of bone metastases as his initial bone scan, with intense uptake in the sacral ala (SUV of 22).
While a primary focus of incorporating SBRT into the treatment paradigm has been to safely reduce the treatment burden, without adversely impacting/worsening the treatment side effect profile compared to conventional fractionation regimens, there is evidence that SBRT may be an ‘antalgic’ treatment approach. In an open-label, multicentre, randomized, controlled, phase 2/3 trial published by Sahgal et al. in Lancet Oncology in 2021, administration of SBRT to 229 patients with MRI-confirmed spinal metastasis, with no more than three consecutive vertebral segments included in the treatment volume, was associated with improved pain outcomes compared to conventional external beam radiotherapy. At 3 months, a complete response for pain was observed in 35% of SBRT patients, compared to 14% of those in the conventional external beam radiotherapy-treated group. This benefit was maintained at the 6-months assessment (32% versus 16%).1 Thus, when considering SBRT for these patients, it is important to be cognizant of the potential pain/health quality of life benefits, in addition to potential oncologic benefits.
What is the evidence for SBRT as a metastases-direct therapy treatment approach in the hormone-sensitive setting? While there are numerous trials, as summarized in the table below, the strongest evidence to date comes from the phase II STOMP (Ost et al.) and ORIOLE (Philips et al.) trials.2,3
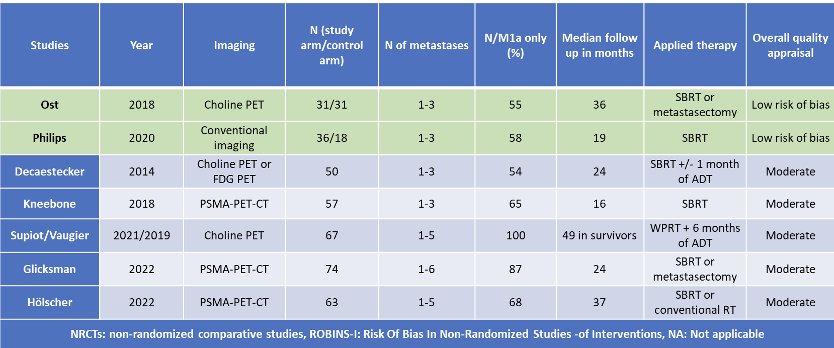
The STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of metastasis-directed therapy for patients with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence with testosterone >50 ng/ml (i.e. metachronous, oligometastatic hormone sensitive prostate cancer). Between 2012 and 2015, 62 patients were randomized 1:1, and metastasis-directed therapy was with either SBRT or metastasectomy. The primary endpoint was time to initiation of ADT, initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease. With a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38- 0 .84, log-rank p=0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases. Secondary endpoint of CRPC-free survival at 5 years was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI: 0.35 - 1.09).2
The ORIOLE trial was a randomized phase II trial of 54 men with metachronous, oligometastatic mHSPC (up to three sites), detected on conventional imaging. Between 2016 and 2018, patients were randomized in a 2:1 fashion to receive SBRT or observation. The primary outcome was progression at 6 months, defined as serum PSA increase, progression detected by conventional imaging, symptomatic progression, androgen deprivation therapy initiation for any reason, or death. Progression at six months occurred in 19% of patients receiving SBRT and 61% undergoing observation (p=0 .005). Treatment with SBRT improved median progression-free survival (HR: 0.30; 95% CI: 0.11-0.81; p=0.002). Importantly, among those who underwent additional PSMA-PET/CT imaging, this trial highlights the importance of targeting all 18F-DCFPyL-PET/CT-detected lesions at time of MDT. Patients with untreated lesions had worse 6-months progression rates (38%) compared to those with no untreated lesions (5%, p=0.03). Further, those with untreated sites of disease had higher rates of new metastases (per conventional imaging) at 6 months (63% versus 16%, p = 0.006) and worse median distant metastasis-free survival of 6 versus 29 months (HR: 0.19, p<0.001).
What about evidence in the CRPC setting? In 2022, Pan et al. published the results of a prospective analysis of SBRT to dual-tracer PET/CT-detected metastases (18F-FDG and 68Ga-PSMA) in patients with conventional imaging non-metastatic prostate cancer and early PSA-progression on ADT (PSA ≤2 ng/ml). SBRT was recommended for patients with five or fewer non-visceral metastases (SBRT group). Patients without detectable metastases (N-/M- group) and those who refused SBRT (ADT group) continued to receive ADT. Patients were followed with conventional imaging.
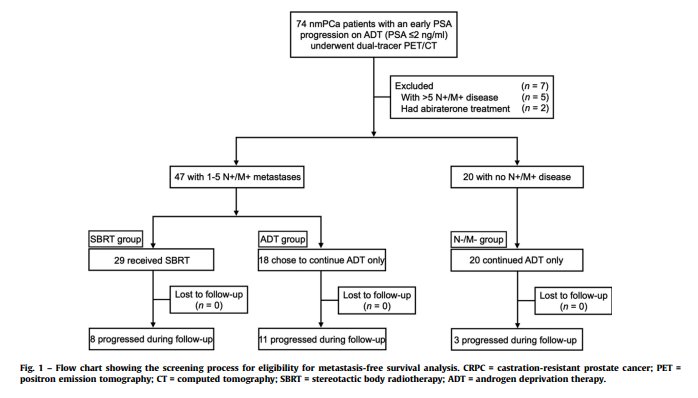
After a median follow-up of 21.4 months, patients in the ADT group had significantly worse metastasis-free survival (MFS; 11 months versus not reached; HR: 4.69, 95% CI: 2.92 – 25, p<0.001). There was no significant difference in median MFS between the SBRT group and the N-/M- group (p=0.26).4
Additional evidence for SBRT in the mCRPC setting comes from ARTO, a multicenter, phase II trial of SBRT in addition to abiraterone plus prednisone in patients with oligometastatic CRPC. This trial included 157 patients, enrolled between January 2019 and September 2022. A biochemical response, defined as a 6-months PSA decrease ≥50% from baseline, was achieved in 92% of SBRT-treated patients, versus 68.3% of patients receiving systemic therapy alone (OR: 5.34, 95% CI: 2.1 to 13.9, p=0.001). SBRT yielded a significant PFS improvement, with a hazard ratio for progression of 0.35 (95% CI: 0.21 to 0.57; p<0.001) in the experimental versus control arm.5

Despite this growing evidence for SBRT across the advanced prostate cancer spectrum, there are still some challenges when selecting the ideal patient for this treatment approach. Physicians need to consider the disease burden and ‘strike a balance’ between the effectiveness of systemic and/or local therapy on survival outcomes.
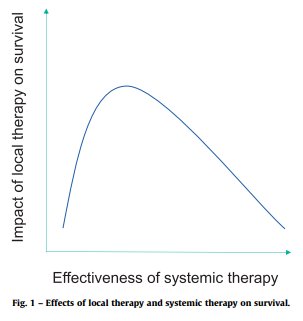
We also need to consider the site of metastasis, with patients harboring visceral disease spread having worse outcomes, and the volume of metastasis.
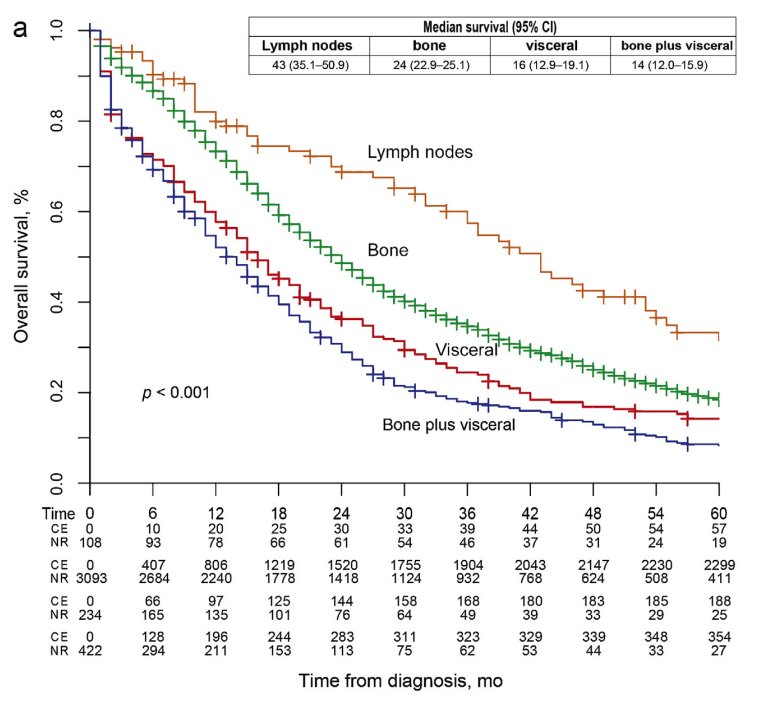
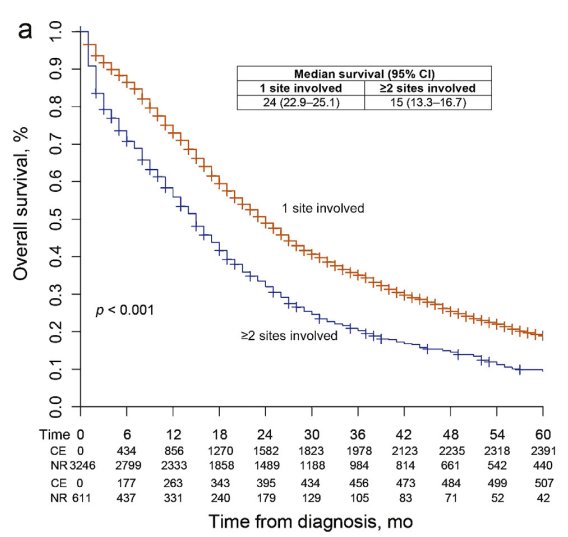
Dr. Fonteyne concluded that:
- Stereotactic radiotherapy for patients with CRPC presenting with pain is an excellent antalgic treatment option
- Stereotactic radiotherapy for patients with CRPC and oligoprogression offers excellent local control, is well-tolerated, and might prolong time to subsequent therapy. However, SBRT remains under investigation, and per Dr. Fonteyne's opinion, should not be performed outside the clinical trial setting.
Presented by: Valerie Fonteyne, MD, PhD, Associate Professor, Radiation Oncology, University of Ghent, East Flanders, Belgium
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Sahgal A, Myrehaug SD, Siva s, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021;22(7):1023-33.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Pan J, Wei Y, Zhang T, et al. Stereotactic Radiotherapy for Lesions Detected via 68Ga-Prostate-specific Membrane Antigen and 18F-Fluorodexyglucose Positron Emission Tomography/Computed Tomography in Patients with Nonmetastatic Prostate Cancer with Early Prostate-specific Antigen Progression on Androgen Deprivation Therapy: A Prospective Single-center Study. Eur Urol Oncol. 2022;5(4):420-7.
- Francolini G, Allegra AG, Detti B, et al. Stereotactic Body Radiation Therapy and Abiraterone Acetate for Patients Affected by Oligometastatic Castrate-Resistant Prostate Cancer: A Randomized Phase II Trial (ARTO). J Clin Oncol. 2023;41(36):5561-8.


