(UroToday.com) The 2024 GU ASCO annual meeting featured an oral prostate cancer session and a presentation by Dr. Karen Hoffman discussing patient-reported health-related quality of life in the randomized FORMULA 509 trial of salvage radiotherapy and 6 months of GnRH agonist with either bicalutamide or abiraterone acetate + prednisone and apalutamide after radical prostatectomy.
Results from FORMULA 509 previously demonstrated that for patients with a PSA >0.5 ng/mL after radical prostatectomy receiving salvage radiation and 6 months of GnRH agonist, the addition of abiraterone acetate + prednisone and apalutamide improved metastasis-free survival compared to bicalutamide (HR 0.32, 95% CI 0.13-0.84):
Physician-reported adverse events among patients randomized to the more intense androgen axis regimen were consistent with the known safety profile of abiraterone acetate + prednisone + apalutamide: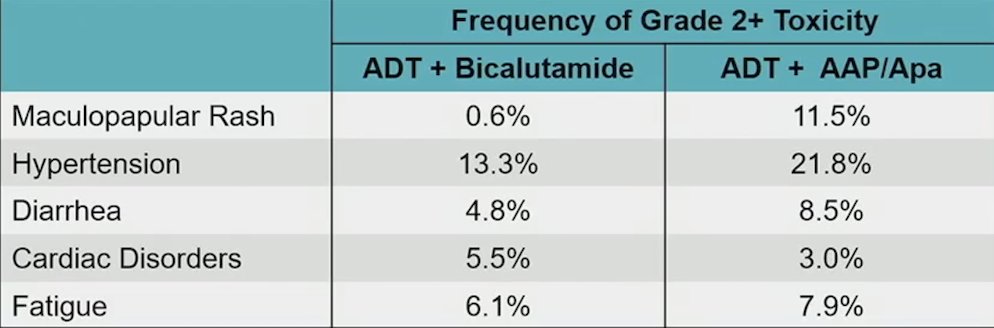
However, it is critical to understand the impact on patient-reported health-related quality of life for these patients. At the GU ASCO 2024 annual meeting, Dr. Hoffman and colleagues presented the patient-reported health-related quality of life results.
Validated questionnaires were administered at baseline, end of treatment, and one year after completion of treatment. These questionnaires included EPIC-26, PROMIS Fatigue, and Saint Louis University Mental Status Exam (SLUMS). EPIC-26 is scored from 0 to 100 with 100 indicating higher function. PROMIS Fatigue is scored using a standardized T score with higher score indicating greater fatigue. SLUMS is scored from 0 to 30, with the following delineation:
- 27 to 30: normal
- 21 to 26: mild neurocognitive disorder
- <=20: dementia
Scores between treatment arms were compared using the t-test. Results were interpreted using established thresholds for clinically meaningful differences (4-6 for EPIC-26 hormonal domain, 5-10 for PROMIS Fatigue).
There were 345 patients randomized to bicalutamide (n = 172) and abiraterone acetate + prednisone/apalutamide (n = 173):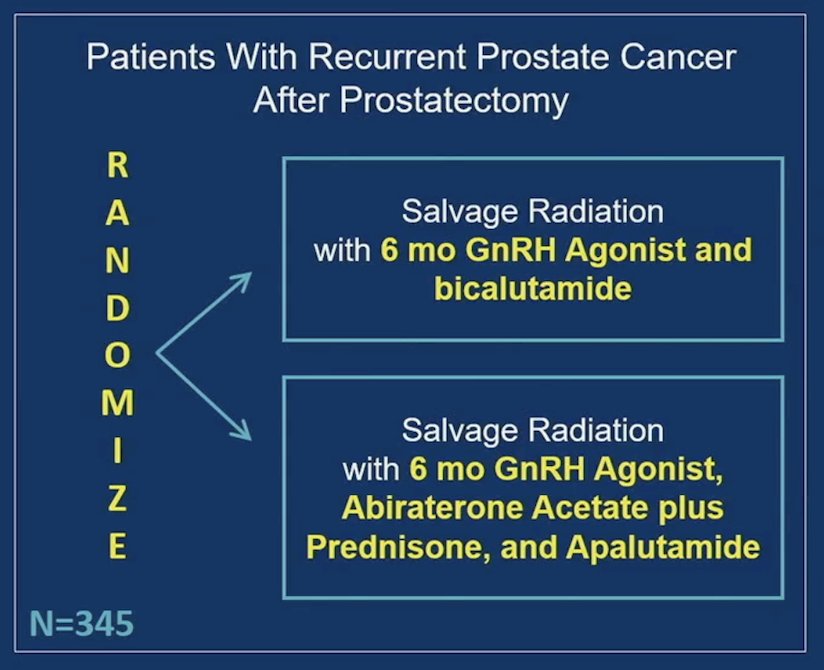
Completion rates at baseline, end of treatment, and 1 year were 96%, 80%, and 70% for EPIC-26, 95%, 79%, and 67% for PROMIS Fatigue, and 96%, 80%, and 70% for SLUMS. There was no meaningful difference in baseline characteristics between those that responded and those that did not respond: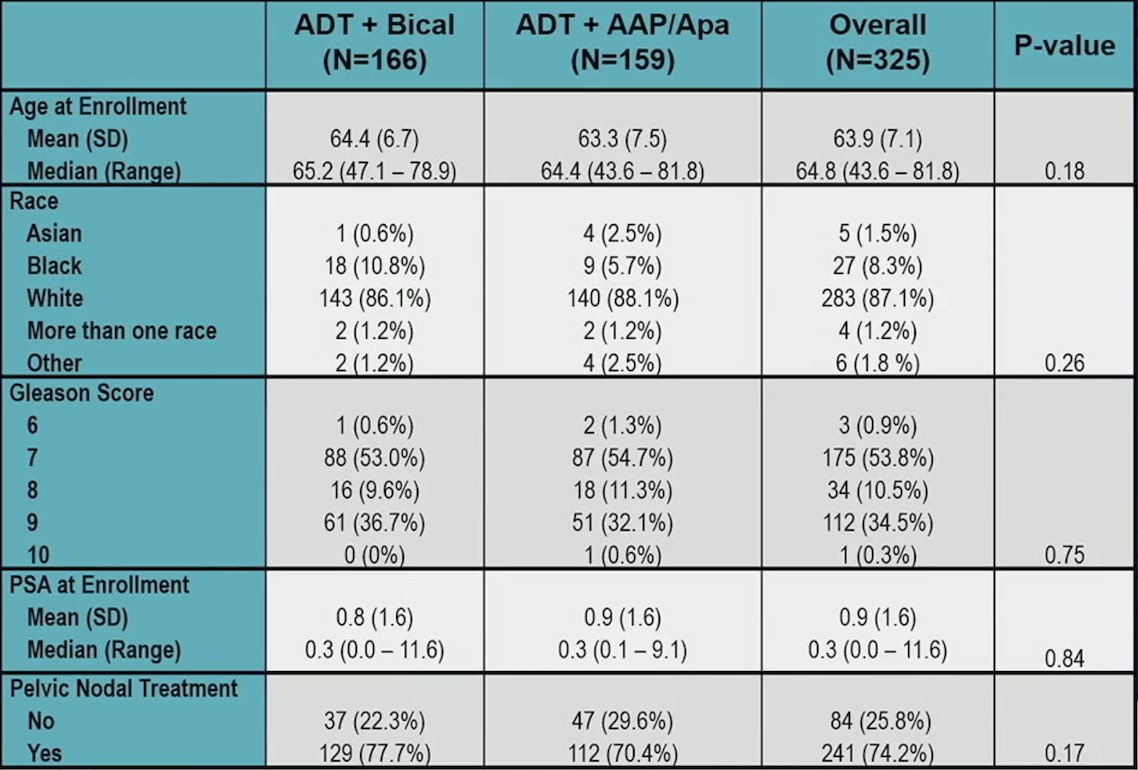
From baseline to end of treatment, both arms demonstrated clinically meaningful declines in EPIC-26 hormonal domain (median change -15 bicalutamide; -15 abiraterone acetate + prednisone/apalutamide) and increase in PROMIS Fatigue (median change 6 bicalutamide; 7.4 abiraterone acetate + prednisone/apalutamide). But, from end of treatment to 1 year after treatment, patient-reported health-related quality of life improved to near baseline for both EPIC-26 hormonal function (bicalutamide median score baseline: 95, end: 75, 1 year: 90; abiraterone acetate + prednisone/apalutamide a baseline: 95, end: 75, 1 year: 90) and PROMIS fatigue (bicalutamide median score baseline: 43.1, end: 48.6, 1 year: 46.0; abiraterone acetate + prednisone/apalutamide baseline: 43.1, end: 51.0, 1 year: 46.0). As follows is the figure for EPIC-26 hormonal domain: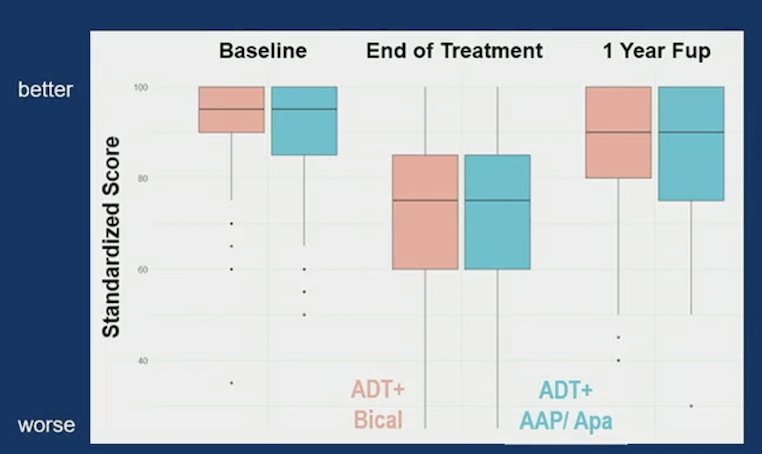
As follows is the figure for PROMIS Fatigue: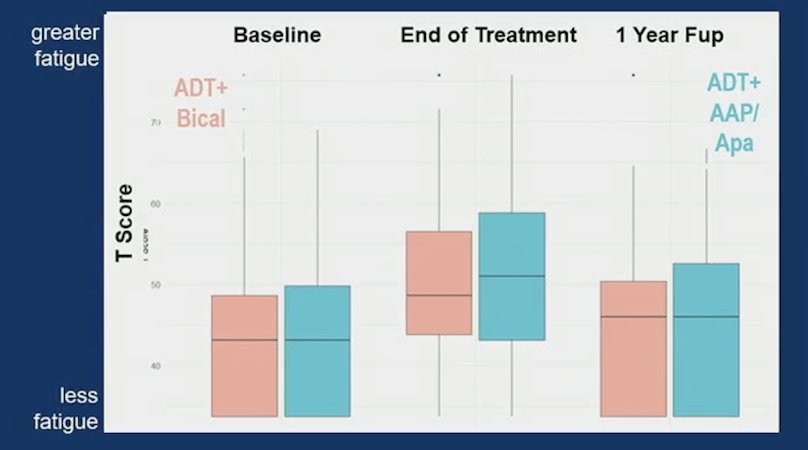
Similar results were also noted for EPIC-26 sexual function, although at baseline only 21% of patients had erections firm enough for intercourse: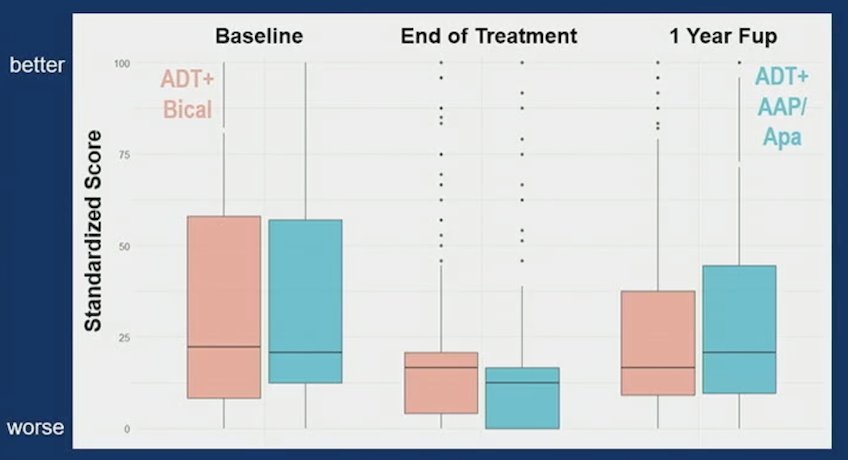
Median SLUMS score was within normal range at baseline (27 bicalutamide vs 27 abiraterone acetate + prednisone/apalutamide), end of treatment (28 bicalutamide vs 28 abiraterone acetate + prednisone/apalutamide) and 1 year after treatment (27 bicalutamide vs 27 abiraterone acetate + prednisone/apalutamide):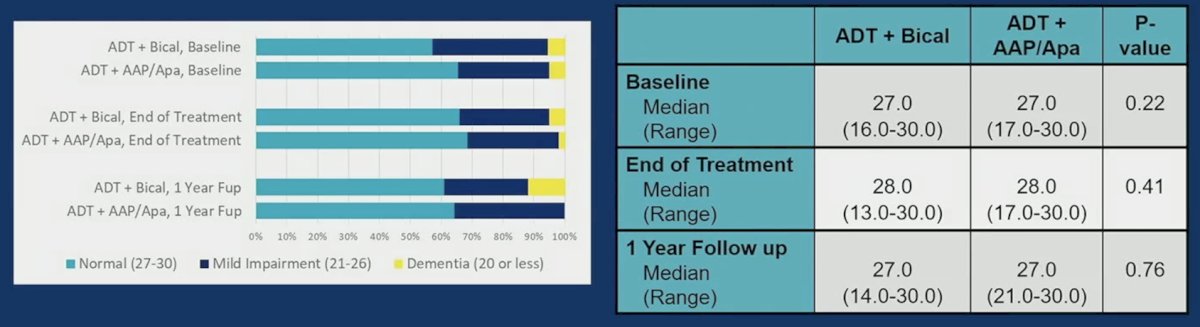
There was no difference in patient-reported hormonal function (p = 0.40), fatigue (p = 0.78), or mental status (p = 0.41) between treatment arms, at end of treatment and 1 year after treatment (p = 0.78; p = 0.89; p = 0.76, respectively).
In the context of comparing the results of the FORMULA 509 trial to the RADICALS-HD trial, RADICALS-HD demonstrated that 24 months of ADT improved metastasis free survival compared to 6 months of ADT. However, in the FORMULA 509 trial intensified arm, 86% of patients recovered their testosterone by 24 months, suggesting better health related quality of life than 24 months of ADT: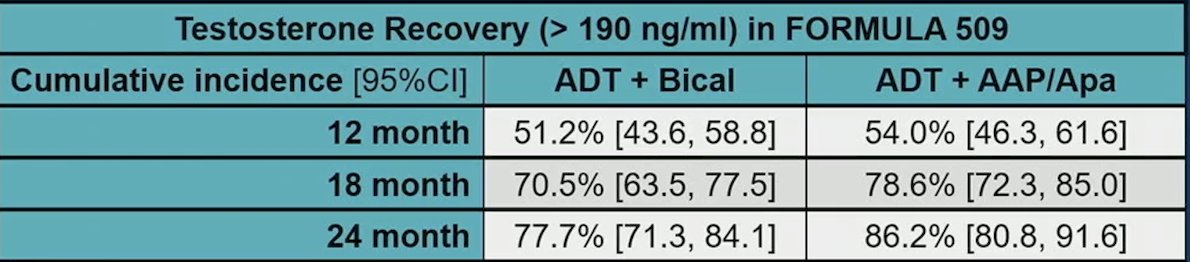
Dr. Hoffman concluded her presentation discussing patient-reported health-related quality of life in the randomized FORMULA 509 trial with the following take-home points:
- In the randomized FORMULA 509 trial, the addition of abiraterone acetate + prednisone + apalutamide to salvage radiotherapy + 6 months of ADT improved oncologic outcomes, without causing a detectable difference in patient reported hormonal function, sexual function, fatigue, or mental status through 1 year after treatment compared to bicalutamide
- Given the favorable patient reported health-related quality of life outcomes, six months of intensified ADT with next generation anti-androgens is an attractive treatment alternative to long duration ADT for patients with rising PSA and unfavorable features after prostatectomy
Presented by: Karen E. Hoffman, MD, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.


