(UroToday.com) The 2024 GU ASCO annual meeting featured a session examining the emerging evidence in localized and recurrent prostate cancer, including a presentation by Dr. Channing Paller discussing systemic treatment of high-risk biochemical recurrence. Dr. Paller started her presentation by defining biochemical recurrence as a rising PSA:
- After radiation: Phoenix definition – an increase in PSA of at least 2 ng/mL above the post radiation PSA nadir
- After prostatectomy: PSA greater than 0.1 ng/mL on two occasions and failed or declined salvage radiation
- No evidence of metastases on conventional imaging
It is important to note that PSMA PET scans have transformed the treatment landscape of biochemical recurrence, which has led to an ongoing reclassification of patients and subsequent modifications to their treatment plans. Moreover, this may now include broadening treatment to potentially include metastasis-directed radiation or next generation hormonal agents. Indeed, there are pros and cons for PSMA PET imaging for biochemical recurrence:
- Pros: (i) earlier and more accurate disease localization, and (ii) PSMA PET directed therapy
- Cons: (i) upstaging leading to over treatment, and (ii) compromised quality of life with an emerging survival benefit
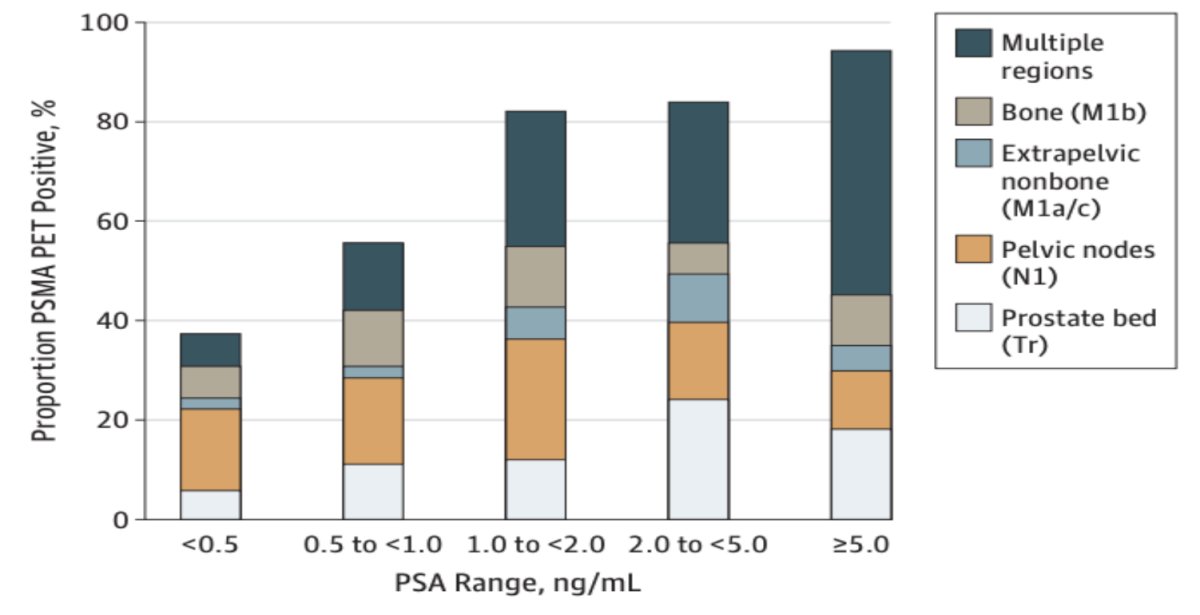
Dr. Paller then highlighted a single arm prospective clinical trial from Fendler et al. that assessed 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer.1 Among 635 patients with biochemical recurrence who underwent 68Ga-PSMA-11 PET, PSMA PET imaging localized recurrent prostate cancer in 75% patients. Detection rates significantly increased with PSA (p < 0.001):
- 38% for <0.5 ng/mL (n = 136)
- 57% for 0.5 to <1.0 ng/mL (n = 79)
- 84% for 1.0 to <2.0 ng/mL (n = 89)
86% for 2.0 to <5.0 ng/mL (n = 158) - 97% for ≥5.0 ng/mL (n = 173)
Historical data suggests that patients with higher Gleason score and rapid PSA doubling times with biochemical recurrence after radical prostatectomy have worse metastasis free survival outcomes, thus portending a high risk population:2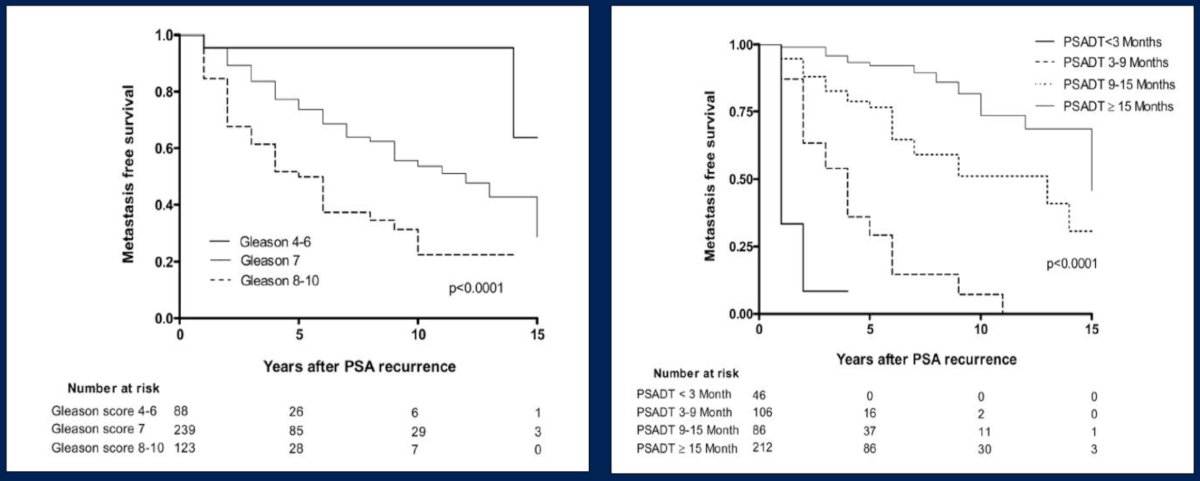
Dr. Paller highlighted the TOAD trial, which assessed when to initiate ADT in patients with biochemical recurrence.3 This was a phase III RCT that randomized 293 men to immediate ADT vs deferred ADT (delay of at least 2 years). Over a median follow-up of 5 years, among patients with biochemical relapse, there was no significant difference in overall survival (HR 0.58, 95% CI 0.30-1.12, p = 0.10). In fact, 41% of patients never started ADT at 5 years after biochemical recurrence: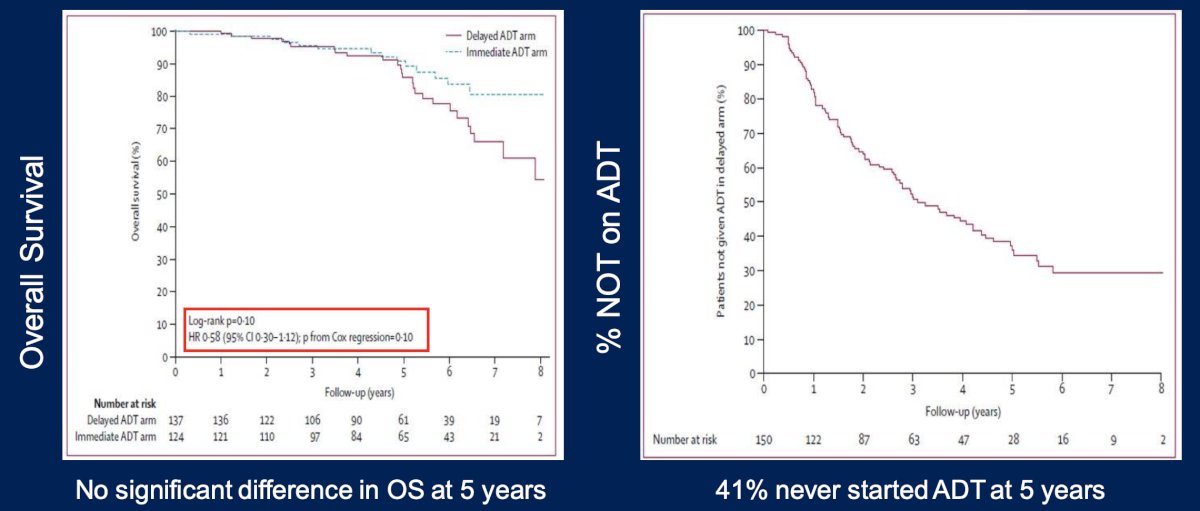
With regards to intermittent versus continuous ADT, Crook et al. published results of an RCT in 2012 assessing continuous versus intermittent (8 month treatment cycles) ADT for patients with biochemical recurrence.4 Between these cohorts, there was no difference in median overall survival (HR 1.03, 95% CI 0.87-1.22) and no difference in disease specific survival (HR 1.18, 95% CI 0.90 – 1.55). Furthermore, the intermittent ADT group had better quality of life scores for hot flashes, libido, and urinary symptoms. The Kaplan Meier curve for overall survival is as follows: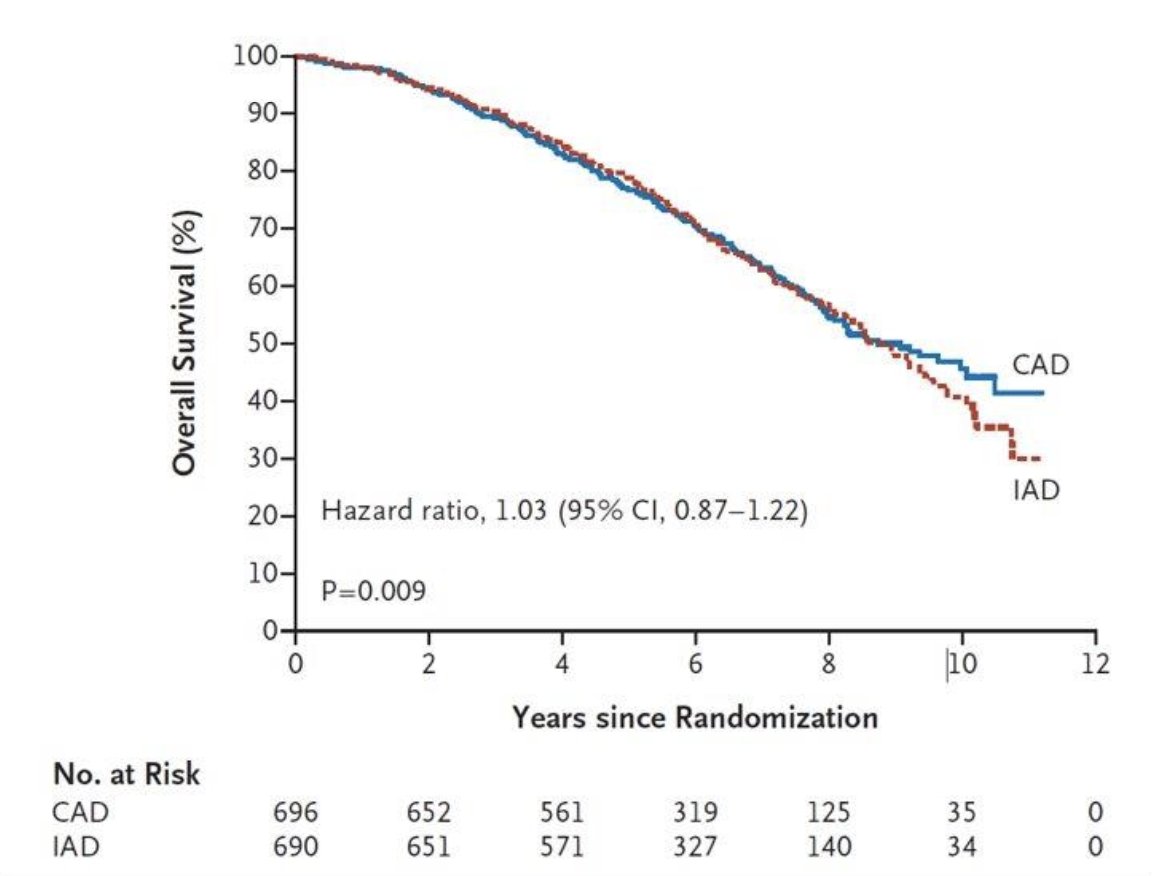
Thus, the aforementioned evidence was the status quo for these patients until November 16, 2023, when the FDA changed this landscape, announcing the approval of enzalutamide for non-metastatic castration sensitive prostate cancer with biochemical recurrence based on results of the EMBARK trial.5 EMBARK was a phase 3 trial that enrolled patients with a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir after primary external beam radiotherapy, with a PSA doubling time of ≤9 months. Patients had no evidence of metastasis on conventional imaging and baseline testosterone was ≥150 ng/dL. Hormone therapy ≥9 months prior to enrolment was permitted. Patients underwent stratified randomization (by PSA level, PSA doubling time, and prior hormonal therapy receipt) to one of three arms:
- Enzalutamide 160 mg (standard dose) + leuprolide (blinded arm)
- Placebo + leuprolide (blinded)
- Enzalutamide monotherapy (unblinded)
PSA was assessed at 36 weeks, and if patients had a PSA < 0.2 ng/mL treatment was suspended at week 37 and PSA was monitored with treatment reinitiated if PSA rose again. If patients had a PSA > 0.2 ng/mL treatment was continued. The primary endpoint was metastasis-free survival, assessed via blinded independent central review, in the enzalutamide + leuprolide versus leuprolide arms only. Key secondary endpoints included overall survival and safety outcomes. The trial schema for EMBARK is as follows: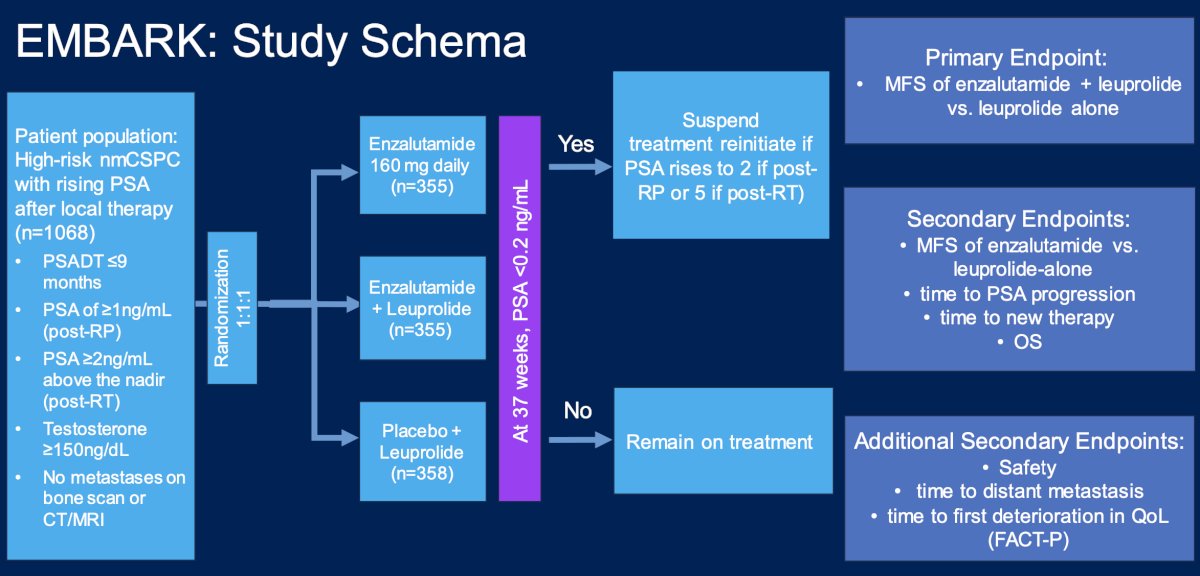
At a median follow-up of 5 years, the combination of enzalutamide + leuprolide versus leuprolide alone demonstrated a significant improvement in metastasis free survival (HR 0.42, 95% CI 0.31 – 0.61, p < 0.0001). The median metastasis free survival was not reached in either arm to date: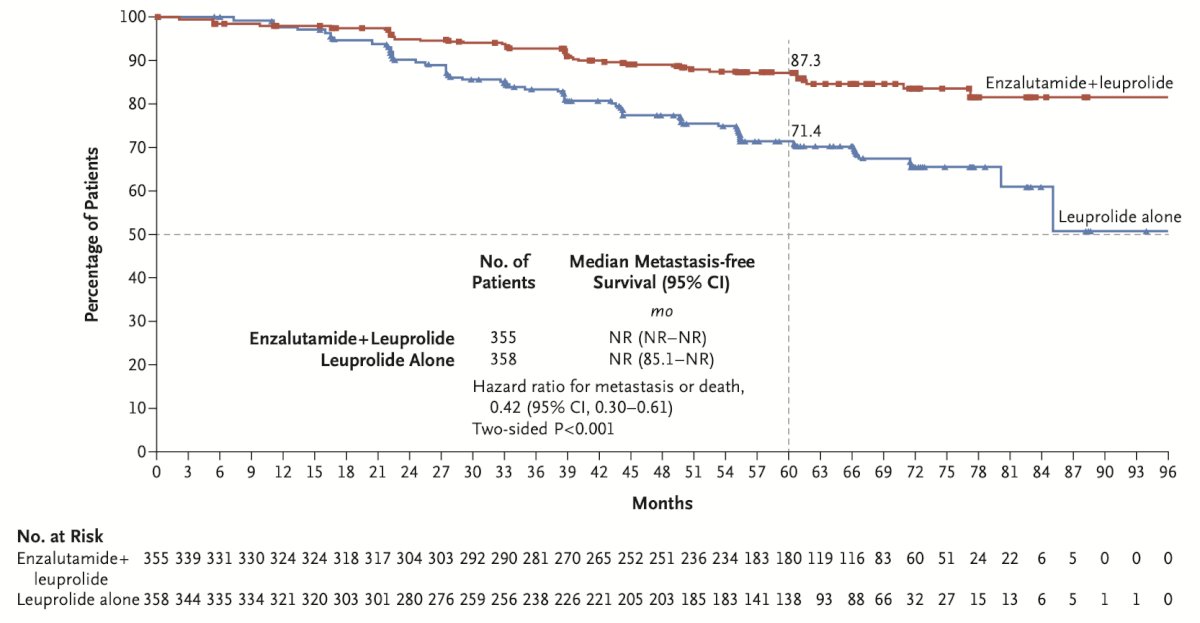
Comparisons between the enzalutamide monotherapy and leuprolide monotherapy arms were also performed. This demonstrated prolonged metastasis free survival in the enzalutamide only arm, with a HR of 0.63 (95% CI 0.46 – 0.87, p = 0.0049):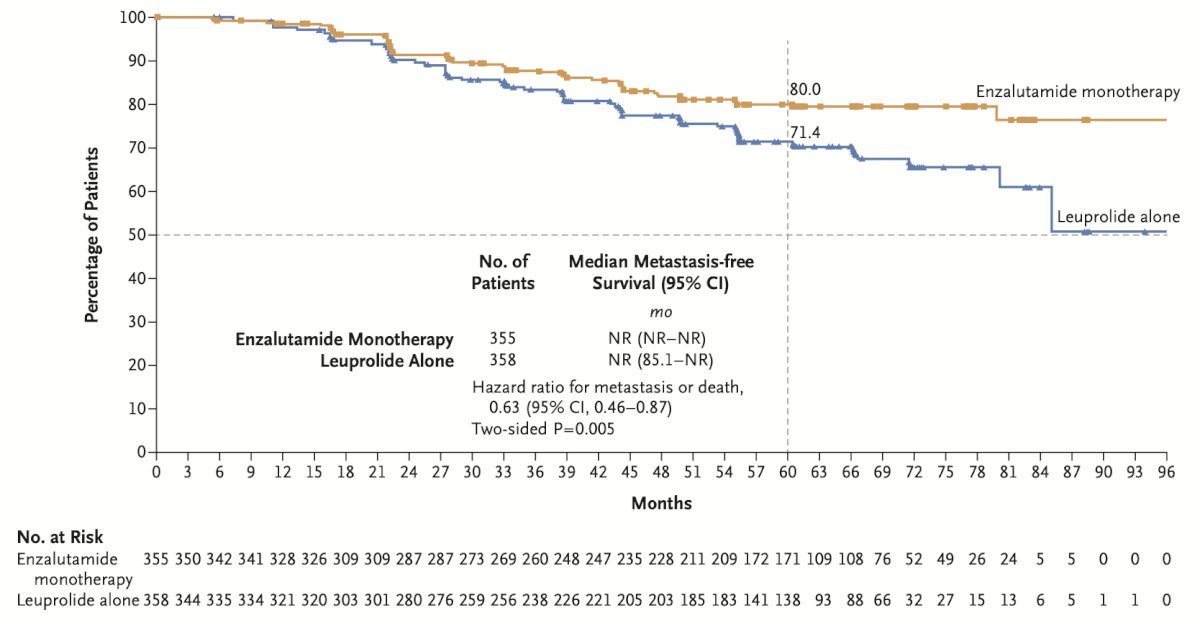
Among the secondary endpoints, generally, these were improved with enzalutamide + leuprolide or enzalutamide alone versus leuprolide alone: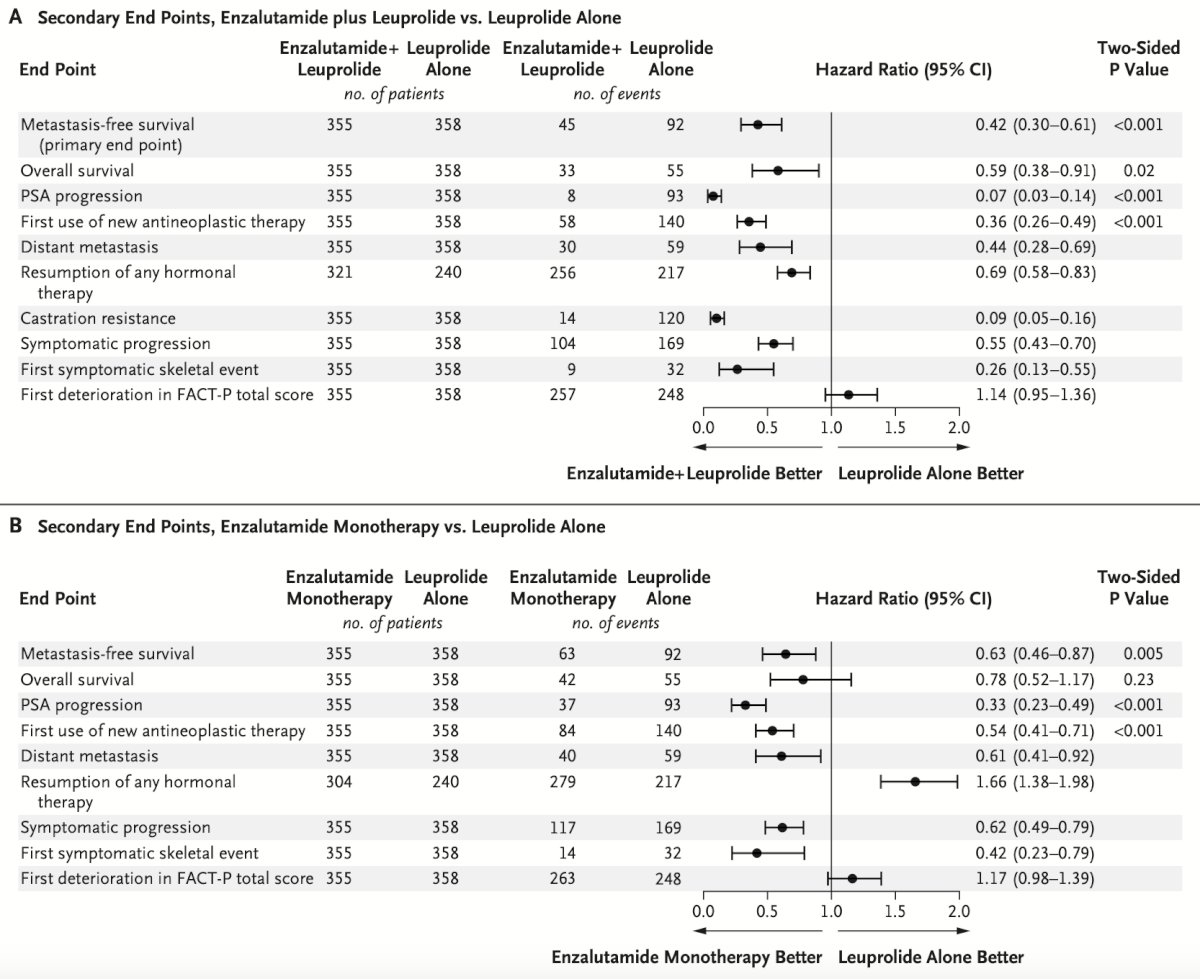
Dr. Paller noted that the most common adverse events leading to study drug discontinuation was fatigue (3% with enzalutamide combination versus 1% and 2% with leuprolide and enzalutamide monotherapy, respectively). Overall, the most common adverse events (>15% of patients) for all treatment cohorts were hot flashes and fatigue.
The combination enzalutamide + leuprolide arm also demonstrated an overall survival improvement (HR 0.59, 95% CI 0.38 – 0.90, p = 0.0142). While the 95% CI upper bound does not cross 1 and the p-value is < 0.05, the overall survival outcome is not yet mature and does not yet meet the pre-specified efficacy boundary of p < 0.0001: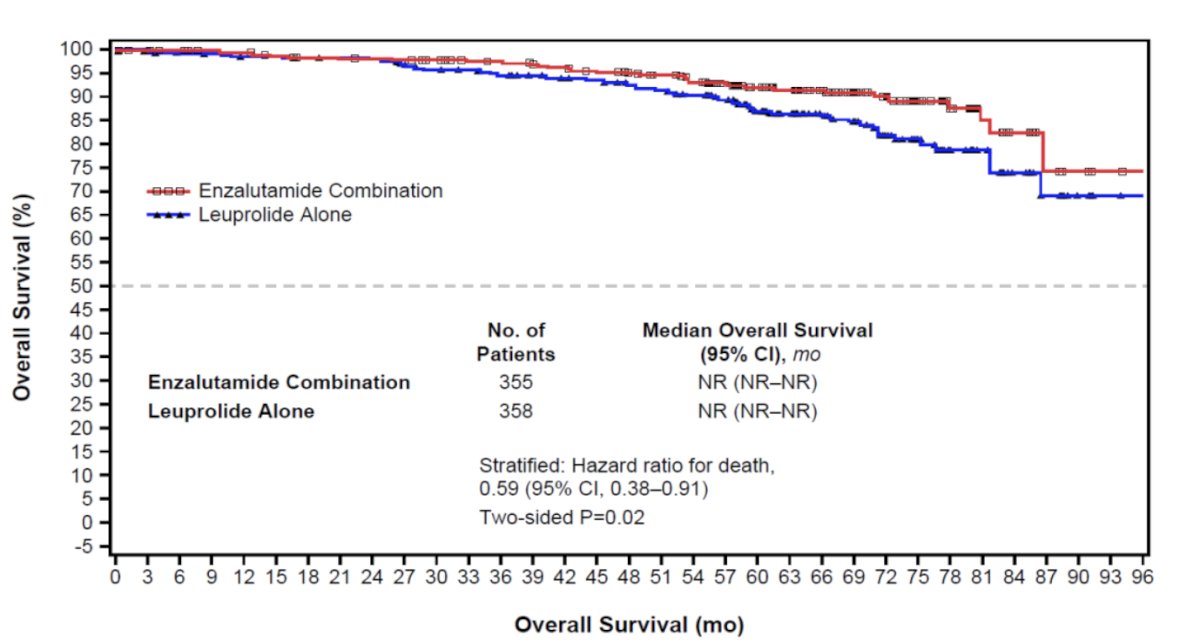
Dr. Paller also noted the patients achieving the outcome of PSA < 0.2 ng/ml at week 36 between the three arms. As seen below (patients suspending treatment), this outcome was observed in 91% and 86% of patients in the enzalutamide + leuprolide and enzalutamide monotherapy arms, respectively, compared to 68% in the leuprolide only arm. The median duration of treatment suspension was, as expected, highest in the enzalutamide combination arm (20.2 months):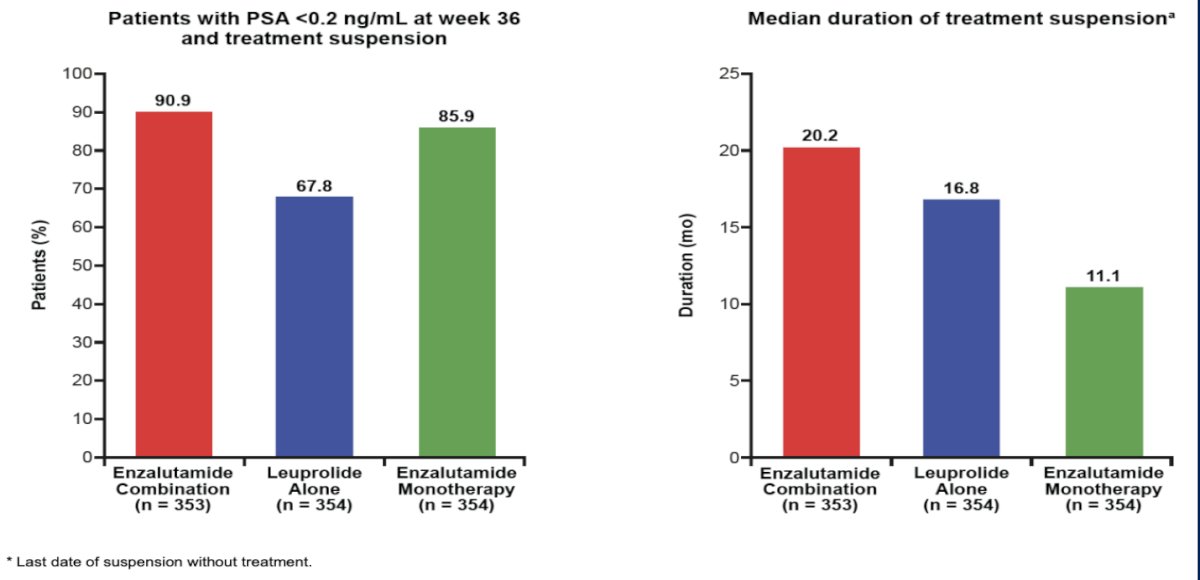
To summarize the EMBARK trial, Dr. Paller made the following statements:
- Enzalutamide is approved with or without an LHRH analog for biochemical recurrence treatment
- The overall survival data looks promising, and we are awaiting maturity of the data
- There are several things to consider when deciding between therapies:
- Metastasis free survival
- Side effects: hot flashes for the enzalutamide + leuprolide combination arm; gynecomastia for the enzalutamide monotherapy arm
- Treatment suspension duration
- Patient comorbidities: seizures, frailty, cognitive/memory impairment, etc
- Drug/drug interactions
Dr. Paller then discussed the phase 3 PRESTO trial, which was an open label study of androgen annihilation in patients with high risk biochemical recurrence. This trial had 3 arms, including Arm A (LHRH analog), Arm B (LHRH analog + apalutamide), and Arm C (LHRH analog + apalutamide + abiraterone acetate + prednisone). The primary endpoint was biochemical progression free survival (PSA > 0.2 ng/mL) for each experimental arm versus the control arm. The trial schema is as follows: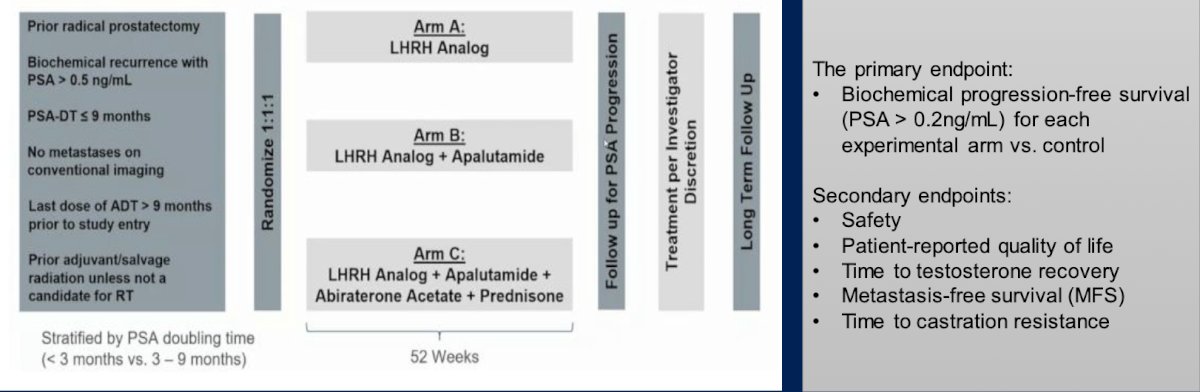
Both experimental arms improved biochemical progression free survival compared to the LHRH analog arm: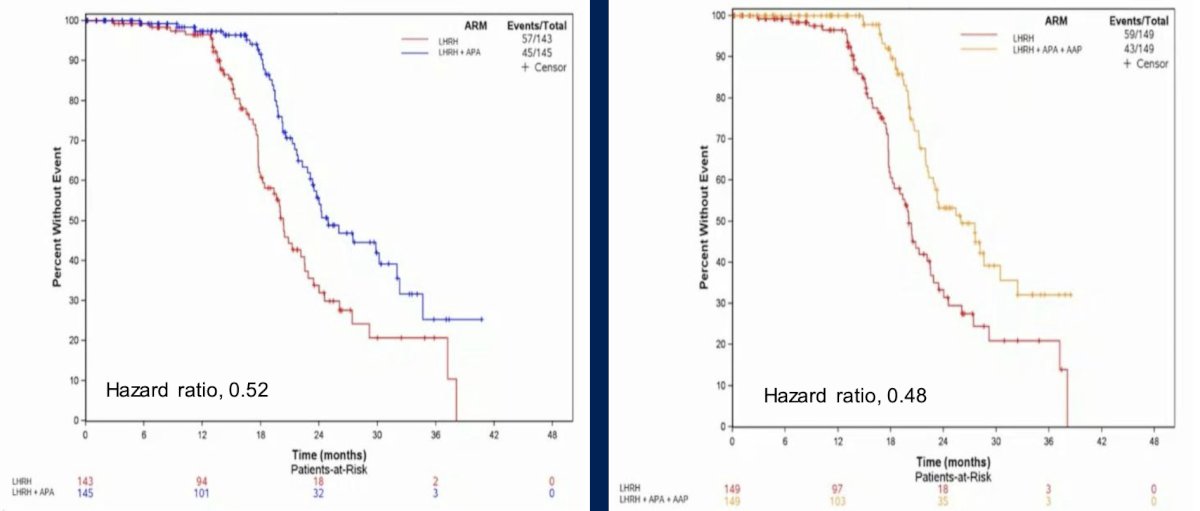
At the time of the interim analysis, there was more complete androgen receptor blockade with apalutamide in addition to ADT prolonging biochemical progression free survival with a manageable safety profile. Additionally, there was more hypertension seen in the abiraterone acetate + prednisone containing treatment arm. As such, to date, the conclusion of this study is that intensification of ADT should be considered in high-risk biochemically relapsed prostate cancer, but there does not appear to be a further benefit with the addition of abiraterone acetate + prednisone to apalutamide.
Dr. Paller’s current treatment algorithm in clinical practice for patients with biochemical recurrence is highlighted as follows: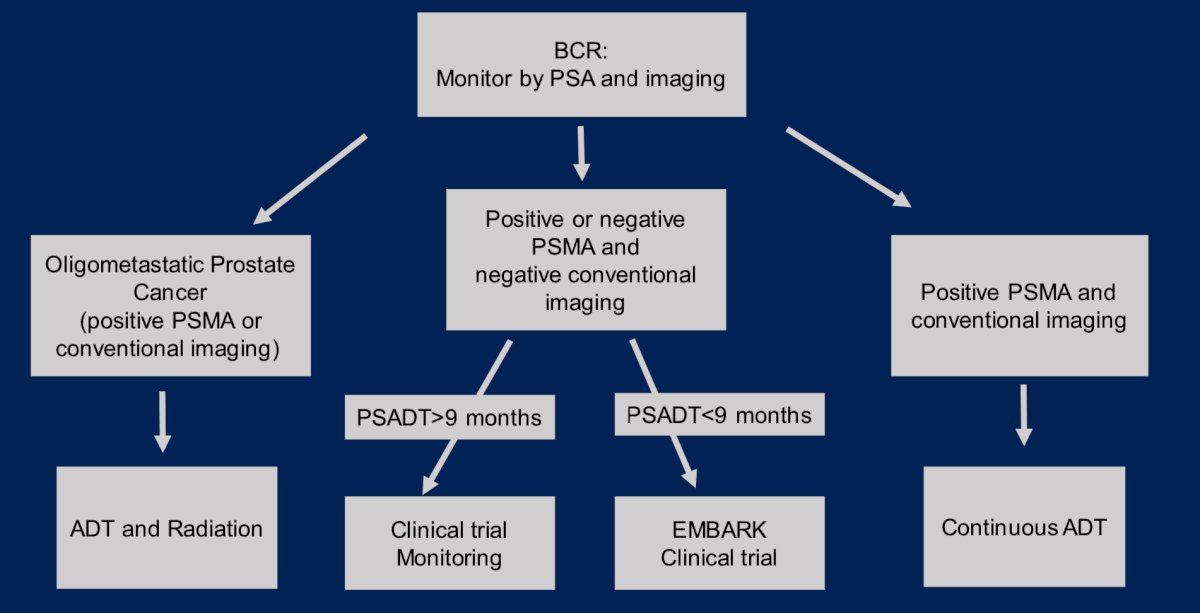
Dr. Paller concluded her presentation discussing systemic treatment of high-risk biochemical recurrence with the following take-home points:
- The FDA has approved enzalutamide in combination with LHRH analog and enzalutamide monotherapy for biochemical recurrence patients with a PSA doubling time <= 9 months
- Clinical trials are an alternative treatment option, such as nivolumab for MMR-deficient prostate cancer with PSA recurrence after local therapy (NCT04019964)
- Based on the EMBARK trial, there is a new standard of care
Presented by: Channing J. Paller, MD, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019 Jun 1;5(6):856-863.
- Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012;109(1):32-39.
- Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomized, multicentre, non-blinded, phase 3 trial. Lancet Oncol 2016;17(6):727-737.
- Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med 2012;367(10):895-903.
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.


