(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to an “Emerging Evidence in Localized and Recurrent Prostate Cancer” session. Dr. Scott Eggener argued in favor of renaming Gleason 3+3 (Grade Group 1 [GG1]) disease, articulating his opinion that public health would dramatically improve if GG1 disease were not called cancer.
Dr. Eggener emphasized that this proposed nomenclature change is not motivated by any underlying financial/economic incentives. In fact, by renaming GG1 disease, this would reduce the number of patients with prostate cancer and thus ‘adversely’ impact financial/economic reimbursements for physicians such as Dr. Eggener. The rationale for this proposed change is that although we have come a long way in managing these patients, we still have huge problems with over-screening, over-diagnosing, and over-treating prostate cancer patients. In toto (diagnosis, surveillance, and treatment), GG1 disease is extraordinarily expensive and unnecessarily ‘tortures’ millions of men worldwide. Dr. Eggener and his colleagues thus hypothesize that public health would dramatically improve if GG1 disease were not called cancer.
Prostate cancer is common among men dying of other causes. Numerous autopsy studies (n=19, including 6,000 men) have consistently demonstrated that autopsy (i.e., incidental) prostate cancer is common in older men and increases in prevalence with age, as summarized in the graph below.1 It is thus clear that many men harbor prostate cancer that does not impact their survival. This same rationale has previously been used to promote the use of active surveillance for GG1 disease – currently considered the guideline-recommended, standard of care approach for such patients.
Dr. Eggener noted that there has been consistent evidence over the years that GG1 disease does not behave like a classical solid organ malignancy:
- 0.28% of GG1 cancers extend beyond the prostate capsule (n=2,500)2
- 0% invade the seminal vesicles (n=2,500)2
- 0% metastasize to lymph nodes (n=14,000)3
- Following surgery, the 15-year risk of dying from prostate cancer is 0% (n=12,000)4
- Per Dr. Eggener, he is “not aware of anyone ever having symptoms, a metastasis, or dying from pure GG1”.
As such, he argued that the name of an entity should reflect its clinical behavior, not the limitations of a diagnostic procedures such as a prostate biopsy.
Dr. Eggener acknowledged the oft-cited paper by Haffner et al in 2013 that demonstrated evidence of a single metastasis from a focus of GG1 disease, although there were countless other metastases from higher-grade cancers.5 As such, she argued that, until proven otherwise, there has never been a death in the ‘history of time’ from pure GG1 disease.
Additional evidence to support the indolent nature of GG1 disease comes from the Sauter et al paper published in European Urology in 2015. In this analysis of 9,600 men undergoing surgery at the Martini Klinik, the authors observed that the biochemical recurrence-free rate for patients with GG1 disease approached 100%.6 Dr. Eggener argued that if the cure rate following treatment for a cancer approaches 100%, there is 100% a need to rethink diagnostic, management, and nomenclature paradigms.
The distinction between benign and malignant disease can be simplified using the Merriam-Webster definitions:
- Benign: of a mild type that does not threaten health or life
- Cancer: a malignant tumor of potentially unlimited growth that expands locally by invasion and systemically by metastasis
Dr. Eggener requested the audience to ask themselves: Based on the available evidence, which one of these two definitions best explains GG1 disease?
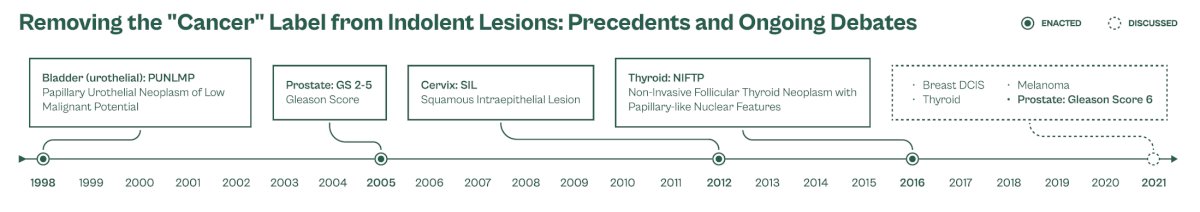
There have been numerous previous precedents where the “cancer” label has been removed from indolent lesions. In Urology, this has been done multiple times:
- Prostate: Gleason 2 – 5 disease
- Bladder: PUNLMP
- Kidney: clear cell papillary (now a tumor, no longer a cancer)
How do specialists feel about renaming GG1 disease? A survey of 1,300 prostate cancer specialists worldwide demonstrated that 39% think it is a ‘good idea’, 30% are ‘uncertain’, and 31% ‘disagree’ with a nomenclature change. The most enthusiastic specialists for a name change were clinicians (urologists, radiation oncologists, medical oncologists versus pathologists), those who are younger (<40 years old), and those who were fellowship trained. Conversely, those who were least enthusiastic were older clinicians and pathologists.7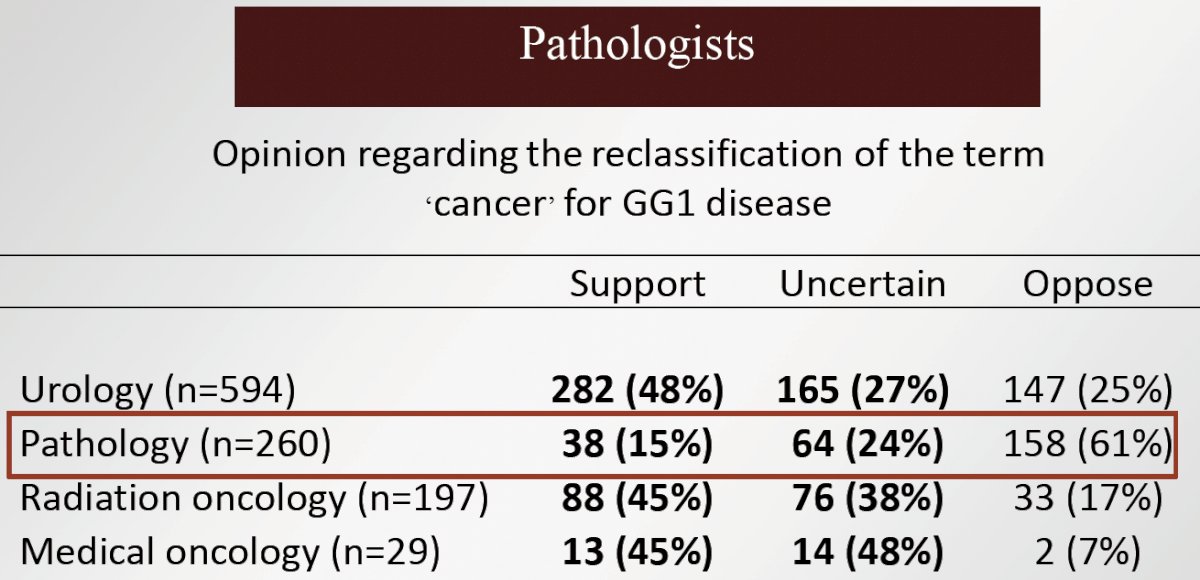
Although pathologists were overall strongly opposed to the proposed nomenclature change, Dr. Eggener highlighted the opinions and rationales of four ‘prominent’ genitourinary pathologists who were in support of considering a name change:
- “Driven by the primary goal of reducing harm to patients”
- “Designation of tumor benignity or malignancy should not be based on the shortcomings of a diagnostic procedure and sampling errors”
- “The optimal approach…..is through multidisciplinary discussions of key stakeholders”
- “If not addressed…will likely continue…as overdiagnosis, overtreatment, and patient's suffering persists”
If we rename GG1 disease, what are some suggested alternatives:
- NLoMP: neoplasm of low malignant potential (51%)
- INeRRT: indolent neoplasm rarely requiring treatment (23%)
- Pre-cancerous prostate lesion (12%)
- IDLE: Indolent lesion of epithelial origin (8%)
- PENIS: prostate epithelial neoplasm of indeterminate significance (0%)
- Acinar neoplasm (suggested by pathologists)
What has become clear is that nomenclature matters. In a recently published report in the Journal of the National Cancer Institute, Berlin et al. published the results of a discrete choice experiment conducted on three cohorts: healthy men, partners, and patients with GG1 (patients). Participants (n=1,254) reported preferences in a series of vignettes with two scenarios each. Across the cohorts, noncancer labels PAN-LMP or prostatic acinar neoplasm of uncertain malignant potential and neoplasm, tumor, or growth were favored over adenocarcinoma and cancer (p<0.01), respectively. Switching adenocarcinoma and cancer labels to PAN-LMP and growth, respectively, increased the preference for active surveillance, versus radical therapy, by up to 17%. Accordingly, the authors concluded that “cancer” labels negatively affect perceptions and decision making regarding GG1. Relabeling (i.e., avoiding the word “cancer”) increases proclivity for active surveillance and would thus likely improve public health.8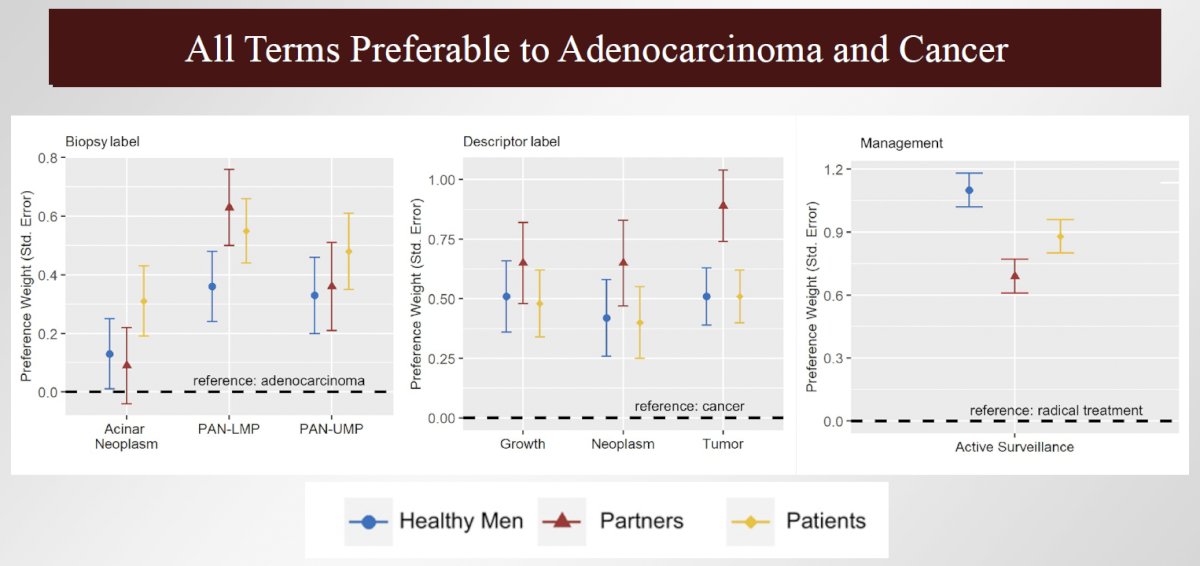
Independent of nomenclature changes, Dr. Eggener acknowledged that there has been a significant evolution in trying to not diagnose GG1 disease. Most experts currently agree that the goal of screening is to identify ≥GG2 disease. Secondary biomarkers (e.g., PHI, 4K, Select MDx, STHLM3) lead to:
- Fewer men requiring a biopsy
- Fewer men diagnosed with Gleason 6 (GG1)
- Diagnosing nearly all the men with Gleason ≥7 (GG2) (compared to biopsy for all)
There are additionally favorable attributes to the now widely adopted MRI, which include:
- Does not routinely visualize GG1
- Intends to visualize GG ≥2 disease
Dr. Eggener argued that even if there is a name change, the recommended follow-up regimens would be similar, with men with erstwhile Gleason 6 (GG1) undergoing:
- PSA (+/- other biomarkers)
- +/- MRI
- +/- biopsy
This is similar to what we already do for men with a:
- Negative biopsy
- ‘Pre-cancerous’ findings such as HGPIN, ASAP, etc.
- GG1 on active surveillance
Dr. Eggener argued that if we significantly reduce overdiagnosis and overtreatment, it is highly more likely that way more men would undergo screening and guidelines would have higher levels of support.
What are the most common ‘counterarguments’ to this proposed nomenclature change?
- Just do active surveillance
- It’s an education problem, not a histology problem
- Microscopically, it meets the definition of cancer
- Genomically, some Gleason 6 have same profile as Gleason ≥7
- Concerns about compliance
- A significant number of these men unknowingly have Gleason ≥7
- This is primarily a United States problem; it does not bother the rest of “us”
Dr. Eggener next went on to address the ‘pitfalls’ of these counterarguments. With regards to ‘just do active surveillance’, Dr. Eggener referenced the recently published AUA Quality (AQUA) registry data published by Cooperberg et al. in 2023 that demonstrated that although the uptake of active surveillance for low-risk prostate cancer is improving, 40% of men with low-risk prostate cancer diagnosed in 2021 received immediate treatment for their disease.9
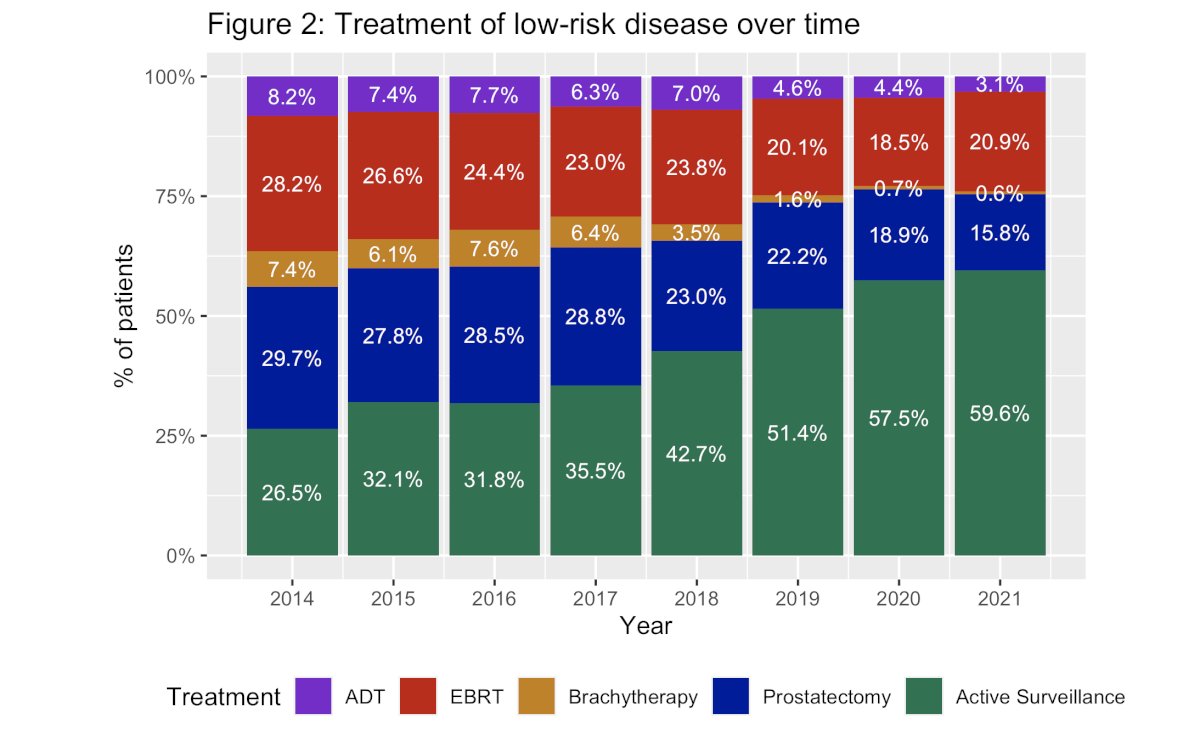
Additionally, a GG1 cancer diagnosis has many downstream effects, including:
- Adversely impacting one’s identity and self-image
- Significantly increasing the risk of suicide (Sweden and the United States)10
- Increasing anxiety (55% of men on active surveillance report cancer-related anxiety)
- Multiple patients in Dr. Eggener’s practice were subsequently unable to obtain life insurance following a GG1 diagnosis
What about ‘it’s an education problem, not a histology problem’? Dr. Eggener noted that there has been excellent data with progressively longer follow-up, and despite trying to educate for more than a decade, there are still 40% of men with GG1 disease in the United States that still receive immediate treatment. Tens of thousands of men every year in the United States alone are undergoing unnecessary treatment, and it is simply not working, per Dr. Eggener.
Next, what about the histologic counterargument that ‘microscopically, it meets the definition of cancer’? Dr. Eggener argued that the clinical reality is what patients care about (not histologic, genomic, or molecular) – will it cause symptoms or problems? Cancer was first defined by Hippocrates 2500 years ago and the microscope invented 400 years ago – we need a 21st century definition. He questioned why is a lack of basal cells the sine qua non of a definitive diagnosis? We’ve changed the definition many times in prostate and other organs, and as quoted by Dr. David Penson, “Clinical pragmatism should prevail over biologic orthodoxy”.
Dr. Eggener did acknowledge the counterargument that some Gleason 6 neoplasms have the same genomic profile as Gleason ≥7 (GG2) tumors. However, in all active surveillance series with superb long-term outcomes, a significant minority had GG1 which were genomically high-risk and still did great. Additionally, there are pre-cancerous lesions (e.g., HGPIN) that share a genomic profile with higher-grade lesions. Dr. Eggener proposed that, alternatively, perhaps ‘high-grade’ lesions that share similar genomic profiles to Gleason 6 (GG1) should be managed with surveillance (or not called cancer).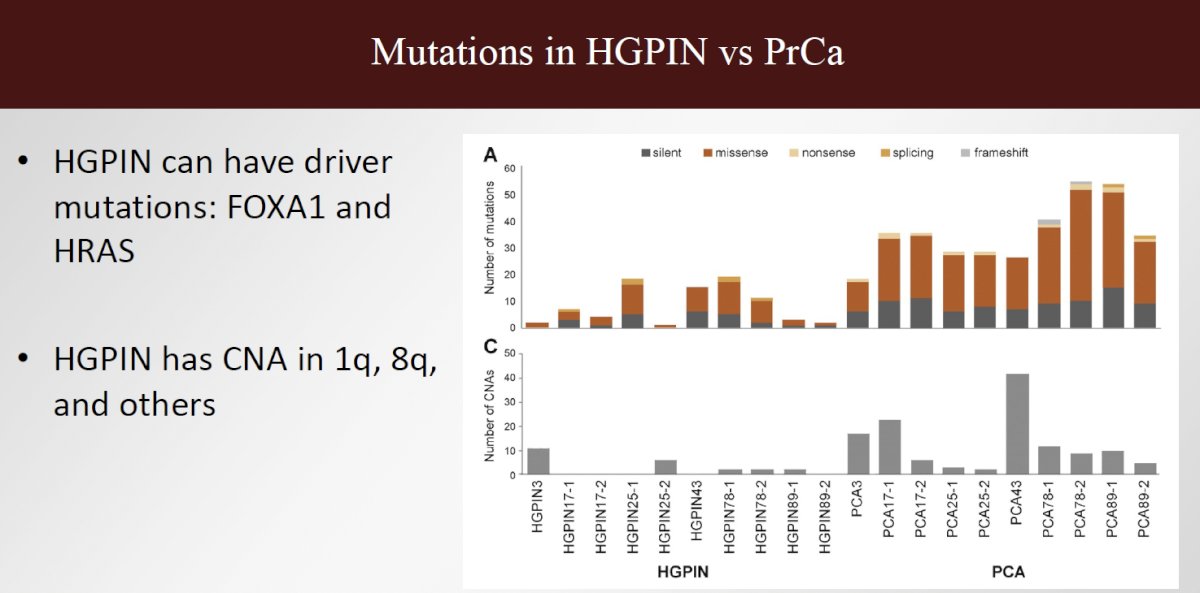
Dr. Eggener also acknowledged that he shares the same concerns regarding compliance. However, he noted that urology practices are full of patients with elevated PSA levels, prior negative biopsies, HGPIN, ASAP, atypia, etc. How are these patients inherently different from those with GG1 disease and how would that impact compliance?
With regards to the counterargument of ‘a significant number of these men unknowingly have Gleason ≥7’, Dr. Eggener argued that this is a sampling issue – are there other conditions called cancer based on unsampled areas which might harbor a real cancer? The same concept can be applied to about 20% of men who die of other causes and never knew about their prostate cancer. The issue here is one of sampling, and, if concerned, a cancer ‘label’ is not the answer – physicians and patients should use available tools (e.g., MRI, repeat biopsy, biomarkers) and find the higher-grade cancer, when present.
Finally, while the overtreatment issue of GG1 disease is primarily a United States problem, with rates of surveillance for low-risk disease being roughly 90% or higher in Sweden, United Kingdom, Australia, and Canada, many other countries with unpublished data have excessive treatment rates of >90% (e.g., Germany, Japan). He argued that removing the cancer label would be beneficial in most countries, regardless of surveillance rates.
Dr. Eggener highlighted ongoing efforts to help change the GG1 nomenclature:
- Large-scale modeling (CISNET)
- Prospective clinical trials
- Patient-friendly path reports (in collaboration with the CDC)
- Ongoing discussions/debates with pathologists
- “Direct to consumer” efforts
Dr. Eggener concluded with the following statement: “The name of GG1 (Gleason 6) should reflect its ‘benign’ or ‘pre-cancerous’ nature. Individual men’s and public health would dramatically improve.”
Presented by: Scott E. Eggener, MD, Bruce and Beth White Family Professor of Surgery, Department of Urology, University of Chicago, Chicago, IL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int J Cancer. 2015;137(12):2795-802.
- Anderson BB, Oberlin DT, Razmaria AA, et al. Extraprostatic Extension Is Extremely Rare for Contemporary Gleason Score 6 Prostate Cancer. Eur Urol. 2017;72(3):455-60.
- Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes? Am J Surg Pathol. 2012;36(9):1346-52.
- Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185(3):869-75.
- Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123(11):4918-22.
- Sauter G, Steurer S, Clauditz TS, et al. Clinical Utility of Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. Eur Urol. 2015;69(4):592-8.
- Saoud R, Woranisarakul V, Paner GP, et al. Physician Perception of Grade Group 1 Prostate Cancer. Eur Urol Focus. 2023;9(6):966-73.
- Berlin A, Ramotar M, Santiago AT, et al. The influence of the “cancer” label on perceptions and management decisions for low-grade prostate cancer Get access Arrow. J Nat Cancer Inst. 2023;115(11):1364-73.
- Cooperberg MR, Meeks W, Fang R, et al. Time Trends and Variation in the Use of Active Surveillance for Management of Low-risk Prostate Cancer in the US. JAMA Netw Open. 2023;6(3)”e231439.
- Guo Z, Gan S, Li Y, et al. Incidence and risk factors of suicide after a prostate cancer diagnosis: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2018;21(4):499-508.


