(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium featured a prostate cancer session and a discussant presentation by Dr. Kim Chi discussing unlocking combination therapy for mCRPC. For this presentation, Dr. Chi highlighted the abstract presented by Dr. Neeraj Agarwal titled “CONTACT-2: Phase 3 study of cabozantinib + atezolizumab vs second novel hormonal therapy in patients with mCRPC,” as well as the abstract presented by Dr. Maha Hussain titled “BRCAAway: A randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with mCRPC bearing HRR mutations.” By way of background for CONTACT-2, Dr. Chi notes that targeting PD-1/PD-L1 for mCRPC as monotherapy has only modest activity in unselected patients. Furthermore, combinations have not improved radiographic progression free survival or overall survival compared to controls in phase III trials:
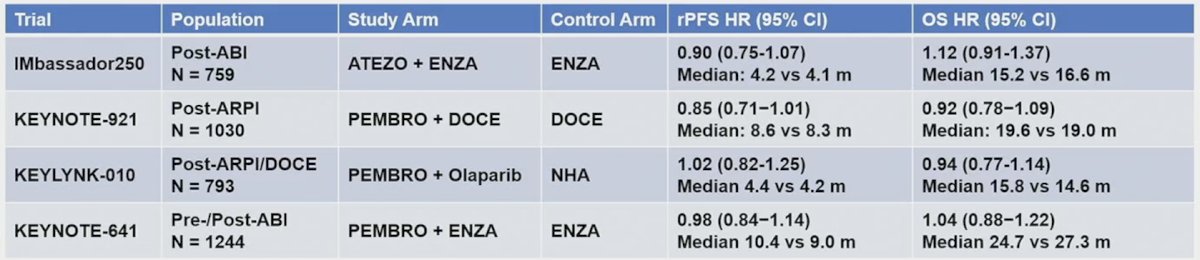
Cabozantinib is an oral multiple tyrosine kinase inhibitor of VEGFR2, c-MET, RET, TAM, and others, which has been tested in COMET-1 in mCRPC noting that monotherapy improved radiographic progression free survival with no difference in overall survival in mCRPC patients after an androgen receptor pathway inhibitor and post-docetaxel:1
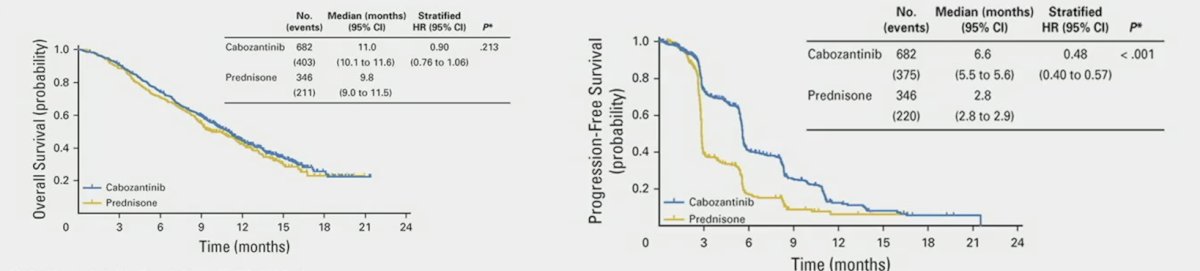
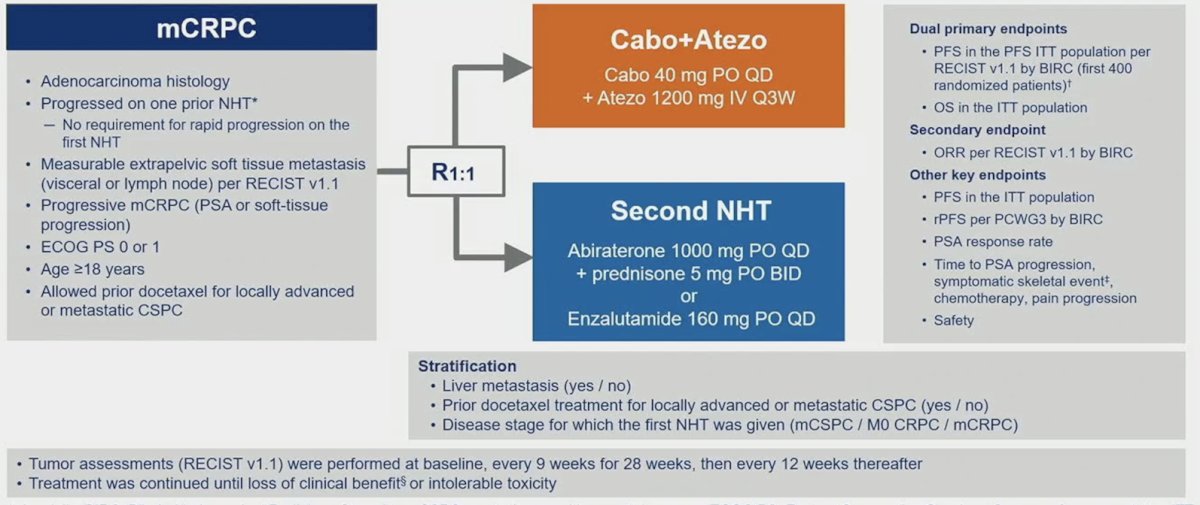
Cabozantinib also has immunomodulatory effects, including reduced myeloid-derived suppressor cells and regulatory T cells and increased PD-L1 expression on regulatory T cells. As such COSMIC-021 was a phase 1b trial testing the combination of cabozantinib + atezolizumab in an mCRPC expansion cohort for measurable disease. This combination resulted in an objective response rate of 23% and median progression free survival of 5.5 months (95% CI 4.3 – 6.6). This led Dr. Chi into his discussion of the CONTACT-2 trial, a phase 3 trial of cabozantinib + atezolizumab vs second novel hormonal therapy in patients with mCRPC. Patients were randomized 1:1 to cabozantinib + atezolizumab (cabozantinib [40 mg PO daily] + atezolizumab [1200 mg IV every 3 weeks]) or control (abiraterone [1000 mg PO daily] + prednisone [5 mg PO twice daily] or enzalutamide [160 mg PO daily]) and were stratified by liver metastasis (yes/no), prior docetaxel for mCSPC (yes/no), and prior novel hormonal therapy for mCSPC, M0CRPC, or mCRPC. The trial design for CONTACT-02 is as follows:
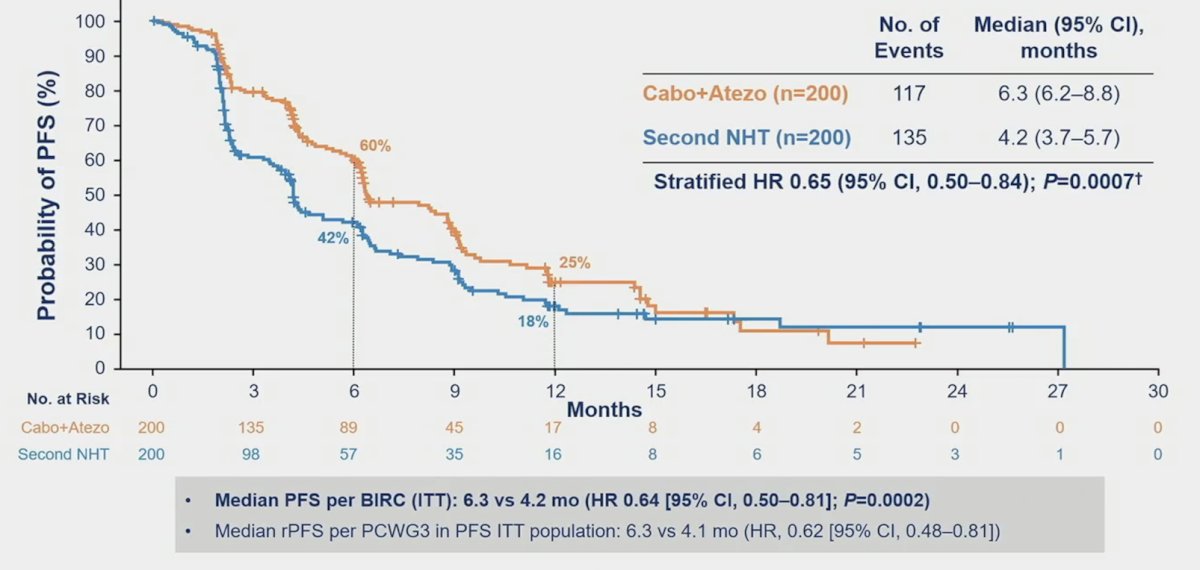
The median follow-up was 12.0 months for all randomized patients and 14.3 months for the first 400 patients. The median radiographic PFS was significantly longer with cabozantinib + atezolizumab vs control (6.3 vs 4.2 months; HR 0.65, 95% CI 0.50-0.84):
The objective response rate was higher in cabozantinib + atezolizumab vs control in patients with follow-up ≥6 months in all randomized patients (14% vs 4%):
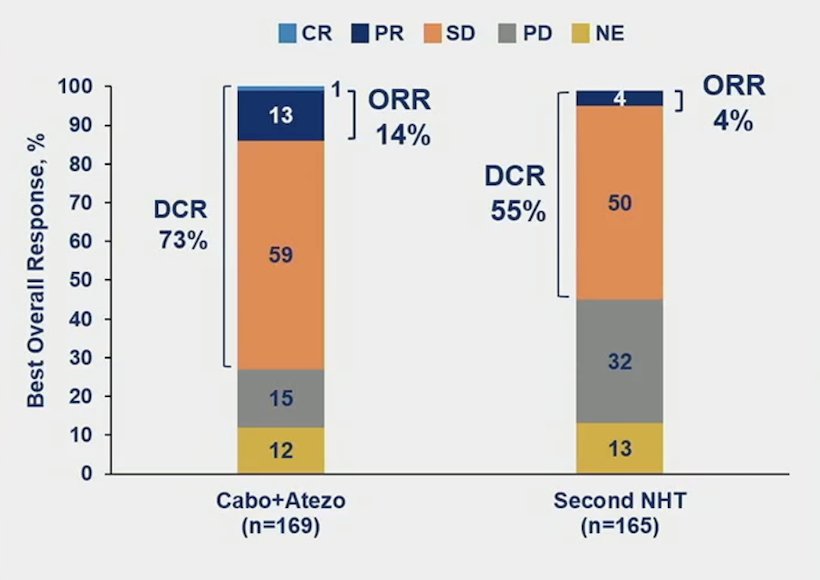
Dr. Chi notes that this was a positive trial, and also the first for an immunotherapy combination in mCRPC, with results consistent with the phase 1b outcomes. However, Dr. Chi notes several limitations of the CONTACT-2 trial:
- The selected patient population had a ~40% screen failure rate
- The absolute radiographic progression free survival benefit is modest and in the absence of other improvements:
- The difference in median radiographic progression free survival is less than one scanning cycle
- There is no difference in overall survival and patient reported outcomes: pain progression ~4 months, and quality of life deterioration ~2 months
- What is the contribution of the individual drugs to the overall benefit? Cabozantinib monotherapy had a radiographic progression free survival of 6.6 months in COMET-1
- Androgen receptor pathway inhibitor switch is not the best standard of care for this patient population with measurable disease and 40% visceral metastases
- Docetaxel has a median radiographic progression free survival of 8-9 months (post-androgen receptor pathway inhibitor)
- Cabazitaxel has a median radiographic progression free survival of 8.0 months (post-androgen receptor pathway inhibitor and post-docetaxel)
- 177Lu-PSMA-617 has a median radiographic progression free survival of 8.7 months in VISION (post-androgen receptor pathway inhibitor and post-docetaxel) and 12 months in PSMAfore (post-androgen receptor pathway inhibitor)
- The adverse event profile is not easy
- Dose delays, holds, and reductions in 40-60% of patients
- If a patient is eligible for cabozantinib + atezolizumab, then they are likely candidates for taxane chemotherapy
Additionally, Dr. Chi notes a low rate of subsequent therapy, thus did these patients with aggressive disease lose an opportunity to get more effective therapy?
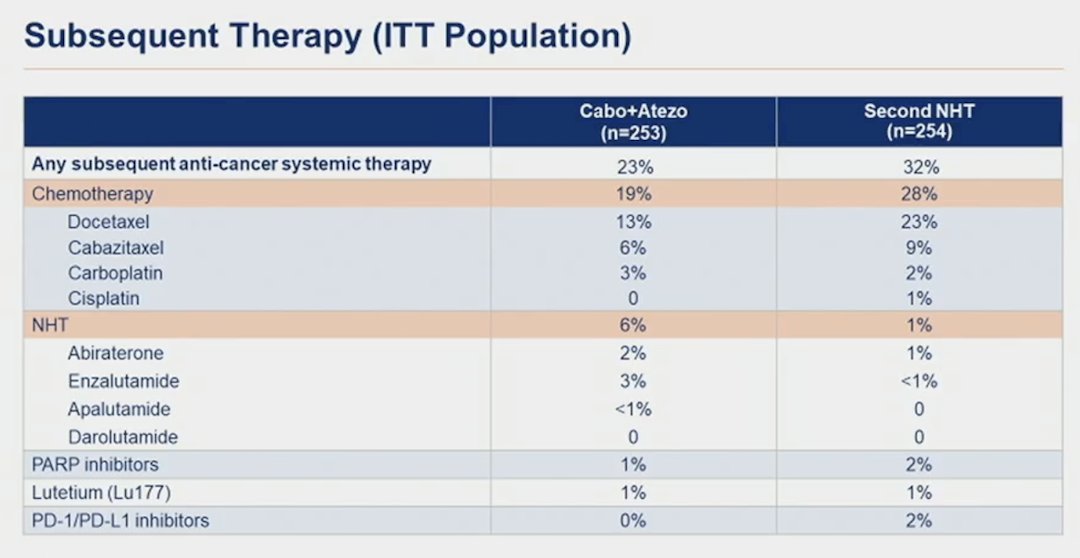
Regarding androgen receptor pathway inhibitor switch, Dr. Chi notes that it can be an appropriate control arm in selected populations, with prolonged responses in patients with a good prognosis. Androgen receptor pathway inhibitor switch in PSMAfore had a median radiographic progression free survival of 5.6 months and 6.0 months in SPLASH. So, is cabozantinib + atezolizumab a new treatment option for mCRPC post-androgen receptor pathway inhibitor? Right now, Dr. Chi cannot recommend this regimen, however additional follow-up and analyses of CONTACT-2 will be informative.
Dr. Chi then discussed the BRCAAway trial, noting that PARP inhibitors are a standard of care treatment for patients with mCRPC and HRR mutations, especially those with BRCA2 mutations. Post androgen receptor pathway inhibitor +/- chemotherapy, PARP inhibitors improve overall survival and radiographic progression free survival, and in androgen receptor pathway inhibitor naïve patients, PARP inhibitors in combination with androgen receptor pathway inhibitors improve radiographic progression free survival versus androgen receptor pathway inhibitor alone. Combination androgen receptor pathway inhibitor + PARP inhibitors have shown improved OS in subgroup analyses, but limited cross-over. Furthermore, these combinations have increased costs, both with toxicity (short and long term) and financial cost. Other the other hand, a sequential strategy could result in a loss of opportunity for treatment.
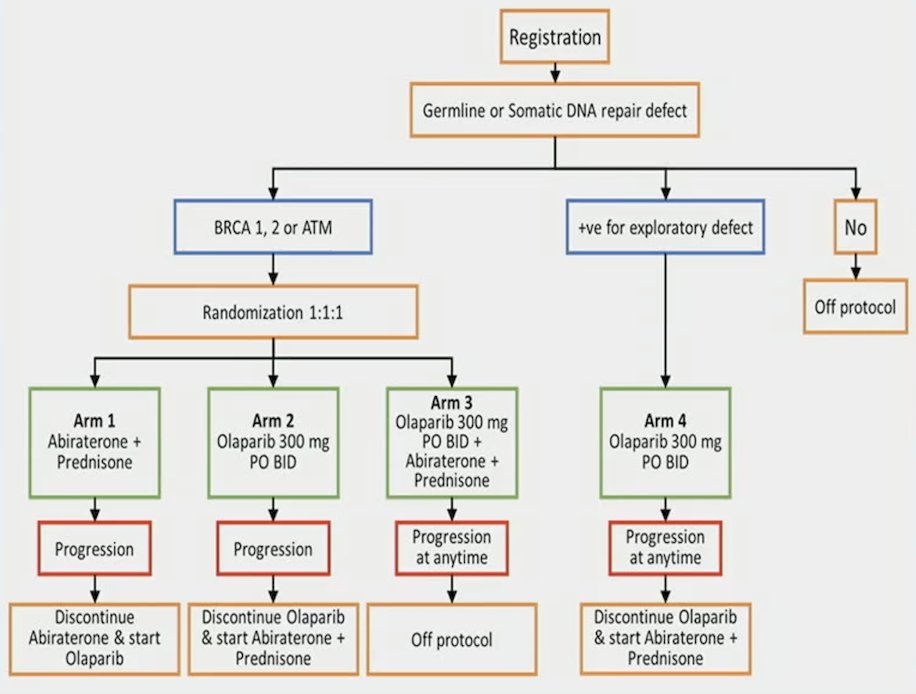
Eligibility for BRCAAway required front-line mCRPC with no prior exposure to PARP inhibitor, AR-inhibitor, or chemotherapy for mCRPC, and a washout period for an antiandrogen, radiation, and other investigational agents. Eligible patients underwent tumor next-generation sequencing/germline testing, and patients with inactivating BRCA1/2 and/or ATM alterations were randomized 1:1:1 to:
- Arm I abiraterone (1000 mg daily) + prednisone (5mg twice daily)
- Arm II olaparib (300 mg twice daily)
- Arm III olaparib + abiraterone/prednisone
The primary endpoint was progression free survival as per RECIST 1.1, PCWG3, clinical assessment, or death. The secondary endpoints included measurable disease response rate, PSA response rate, and toxicity. Arms I and II patients were permitted to cross over at the time of progression. The trial design for BRCAAway is as follows:
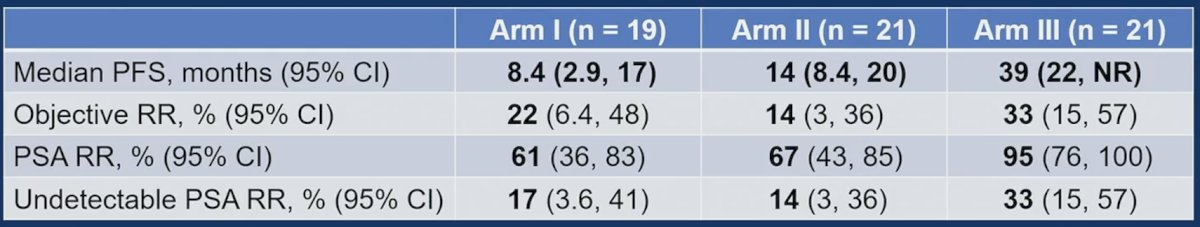
Dr. Chi emphasized that the strengths of this trial design are that (i) there is an olaparib monotherapy arm, (ii) clinical progression was included in the primary endpoint definition, and (iii) crossover design was included. Limitations of the trial design include a small non-comparative study with only 19-21 patients per arm, and most patients had BRCA2 mutations (77%) with an imbalance between arms: Arm 1 – 68%, Arm 2 – 90%, and Arm 3 – 71%. Efficacy results for Arms I-III are presented in the table, with notable improvements in PFS, objective response rate, PSA response rate, and undetectable PSA response rate in Arm III compared to Arms I and II:
Progression free survival, stratified by treatment arm shows a significant benefit for Arm III vs I (HR 0.28, 95% CI 0.13-0.67) and Arm III vs II (HR 0.32, 95% CI 0.14-0.75):
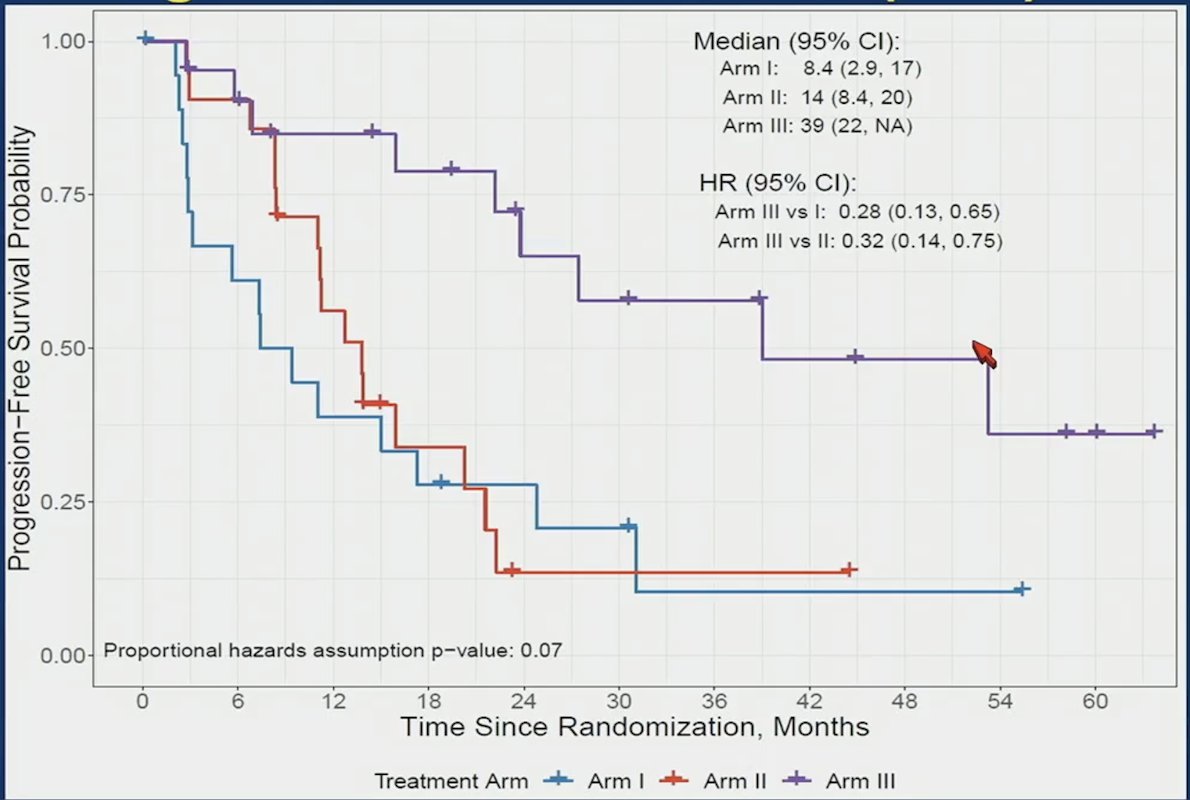
Dr. Chi emphasized that we cannot directly compare crossover patients, given there is a selection bias for this type of analysis. Additionally, less than half of patients crossed over to known effective therapies, which is consistent with real world data showing that many patients do not get more than 1 line of therapy for mCRPC. Moreover, it should be noted that BRCA2 mutated cancers have poor prognosis and can have primary resistance to androgen receptor pathway inhibitors. Overall, Dr. Chi highlighted that the results of BRCAAway support an upfront PARP inhibitor + androgen receptor pathway inhibitor combination as first line therapy for HRR gene mutated mCRPC. This data suggests there is synergy and thus no loss of opportunity. However, this is a small trial, with limited cross over, and is a BRCA2 enriched population.
Dr. Chi concluded his discussant presentation with several take-home points:
- Many mCRPC patients in trials and the real world only get 1-2 lines of therapy
- It is important to select the best treatment upfront
- We need to design trials that ensure (i) control arm therapy that represents best practice, (ii) cross-over where appropriate, and (iii) more data on subsequent therapy (or lack thereof)
Presented by: Kim N. Chi, MD, BC Cancer Vancouver Centre, Vancouver, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Smith, M, De Bono J, Sternberg C, et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J Clin Oncol. 2016 Sep 1;34(25):3005-3013.


