(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium included a prostate cancer session featuring trials in progress and a presentation by Dr. Jose Mauricio Mota discussing the trial design of Daro-PET, a phase 2 trial of darolutamide as a PSMA expression enhancer in patients with localized prostate cancer. PSMA has been explored as a target in imaging studies using PET to unveil occult prostate cancer metastasis and as a target for radioligand therapy. Furthermore, preclinical and retrospective clinical studies suggest that PSMA expression can be rapidly increased by androgen suppression, which might impact the accuracy of disease detection using PSMA-PET scans and radioligand treatment efficacy. In a preclinical model, the use of darolutamide increased PSMA expression in vitro and in vivo, and similarly, treatment with enzalutamide increased PSMA expression after a 7 day treatment in prostate cancer cell lines with low basal PSMA expression.
Daro-PET is a single-arm phase 2 study to evaluate the efficacy of a limited course of darolutamide as a PSMA expression enhancer in men planned for radical prostatectomy due to high-risk localized prostate cancer as per conventional imaging. PSMA PET/CT scans will be acquired before (PSMA-PET 1) and after (PSMA-PET 2) a 7-day treatment with darolutamide 600 mg PO every 12 hours. The trial design for Daro-PET is as follows:
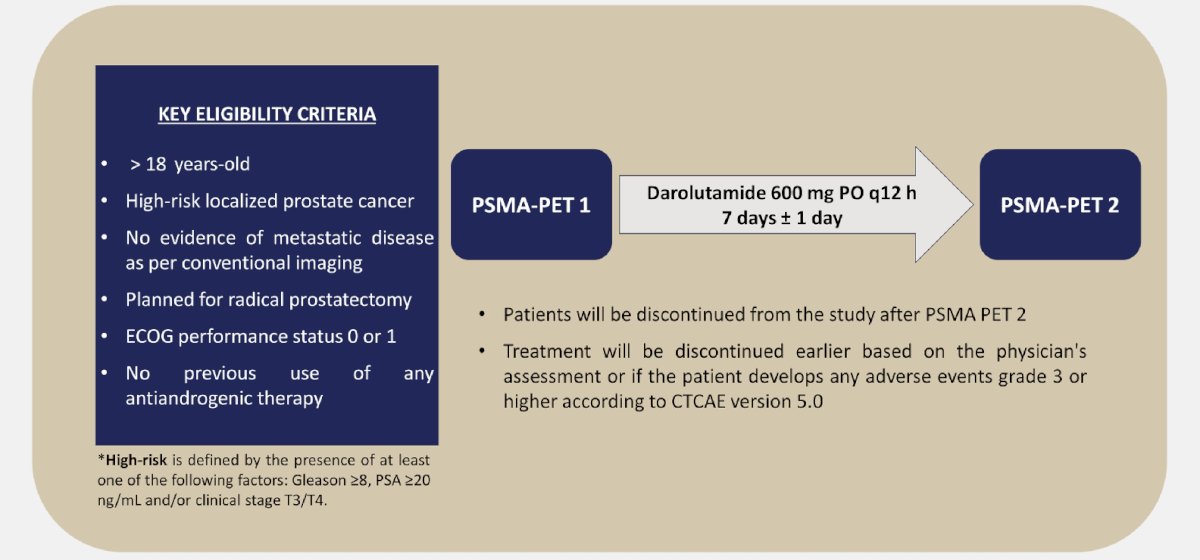
The primary endpoint of this trial is the proportion of patients achieving an increase in SUVmax of 20% or greater from PSMA-PET 1 to PSMA-PET 2.
Key secondary endpoints include:
- Other PSMA PET parameters (for example, SUVmax, SUVmean, total lesion PSMA)
- Proportion of planned management changes
- Detection of pelvic or extrapelvic metastatic disease
- Safety
Correlative exploratory studies will include PSMA expression by immunohistochemistry, gene expression, and methylation patterns between tissue samples from prostate biopsy and prostatectomy. The Daro-PET trial will enroll up to 19 patients using a Simon’s two-stage design, with a power of 0.80 and a one-sided alpha error of 0.05. As of January 9, 2024, 4 of 19 patients have been included in the study.
Clinical trial information: NCT05900973.
Presented by: Jose Mauricio Mota, MD, PhD, Instituto D'Or de Pesquisa e Ensino, Sao Paolo, Brazil
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


