(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer poster session. Dr. Loic Djaileb presented the follow-up analysis of a multicenter, prospective phase 3 imaging trial evaluating pre-surgical 68Ga-PSMA-11 PET/CT for biochemical recurrence risk assessment as a potential surrogate of pelvic lymph node dissection.
With the improved sensitivity of PSMA-PET/CT for the detection of nodal metastases,1 there has been interest in evaluating the use of PSMA-PET/CT as a staging tool to eliminate the need for a staging pelvic lymph node dissection at the time of radical prostatectomy. However, to date, there is insufficient evidence to support such practice, with PSMA-PET/CT having inadequate sensitivity for the detection of small nodal metastases <5 mm in size. But can PSMA-PET/CT performed pre-surgically provide prognostic information for biochemical recurrence? Accordingly, the objective of this study was to compare the prognostic value of pre-surgical PSMA-PET and pelvic lymph nodes positivity (pN1) for biochemical recurrence free-survival in patients with intermediate- to high-risk prostate cancer treated with radical prostatectomy and a pelvic lymph node dissection.
This was an ad hoc analysis of the surgical cohort included in the multicenter, prospective phase 3 imaging trial (n=277; NCT03368547, NCT02611882, NCT02919111). Each 68Ga-PSMA-11-PET/CT scan was interpreted by three blinded independent readers. The risk of biochemical recurrence was assessed using the local histopathology risk score (CAPRA-S, without pN data), presence/absence of extraprostatic disease on PSMA-PET/CT (i.e., cN+/M+), and pN status. The patients were followed for biochemical progression post-radical prostatectomy by the local investigators using data from electronic medical records. Biochemical recurrence was defined by a PSA level >0.2 ng/ml following surgery or initiation of prostate cancer-specific adjuvant/salvage therapy.
A total of 277 patients underwent surgery following the PSMA-PET/CT, and clinical follow-up was available for 240 (87%). The median follow-up from surgery was 32.4 months. A biochemical recurrence event was observed in 91 patients (38%). Evidence of cN1/M1 disease was detected in 17% of patients on PSMA-PET/CT, whereas pN1 disease was observed in 28%.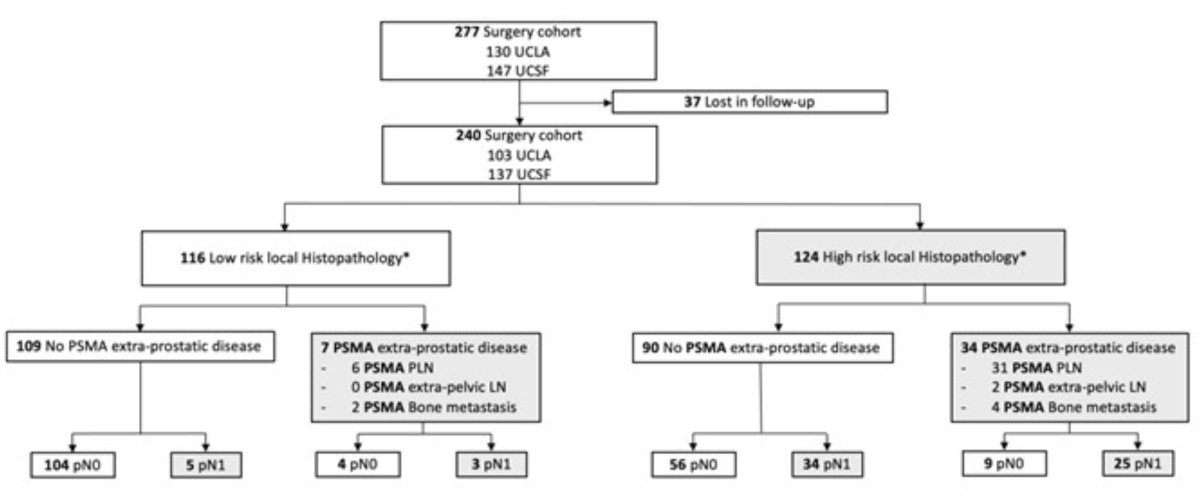
The model combining local histopathology and PSMA-PET performed similarly to that combining local histopathology and pN status for predicting biochemical recurrence (C-statistic: 0.74 versus 0.73, p=0.69).
Among patients with low-risk local histopathology score and PSMA-PET/CT cN0/M0 status, only 5% were pN1. Conversely, among patients with a high-risk local histopathology score and a PSMA-PET/CT positive for cN1/M1 disease, 74% were found to be pN1.
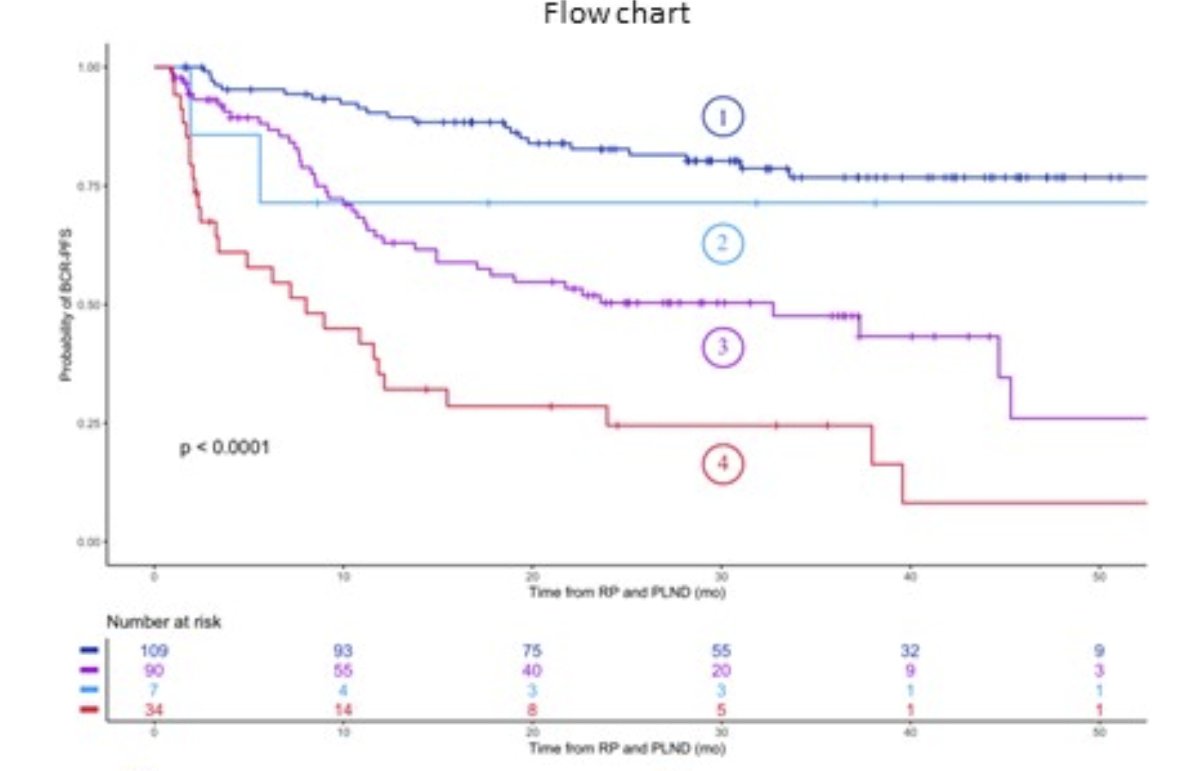

Dr. Djaileb concluded that the combination of pre-surgical PSMA-PET/CT and local histopathology was not statistically different to the reference standard of local histopathology and pN status for the prediction of biochemical recurrence-free survival. Notably, the presence of low histopathology risk combined with absence of cN+ or cM+ disease on PSMA-PET correlated well with absence of pN1 disease, and a positive PSMA-PET/CT combined with a high histopathology risk was highly correlated with presence of pN1 disease.
Presented by: Loic Djaileb, MD, PhD, Visiting Associate Professor, Nuclear Medicine, Department of Molecular and Medical Pharmacology, Ahmanson Translational Theranostics Division, University of California, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.


