(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer rapid oral abstract session. Dr. Himisha Beltran presented the interim results from a phase 1/2 study of HPN328, a tri-specific, half-life extended Delta-like protein 3 (DLL3)-targeting T-cell engager, in patients with neuroendocrine prostate cancer and other neuroendocrine neoplasms.
Dr. Beltran noted that DLL-3 expression increases across the prostate cancer continuum. DLL3+ is non-expressed in benign cells and has minimal expression in prostate cancer cells (0.52%). However, DLL-3 expression increases to 12.5% in castrate-resistant adenocarcinoma and to 77% in neuroendocrine, de-differentiated castrate-resistant prostate cancer.1
HPN328 is a DLL3-targeting T-Cell Engager with strong activity in DLL3+ cancer models and has demonstrated anti-tumor activity in vivo. When DLL3+ patient-derived neuroendocrine prostate cancer xenografts were injected into mice models, HPN328 co-injected with human T cells resulted in tumor regression and prolonged survival of mice.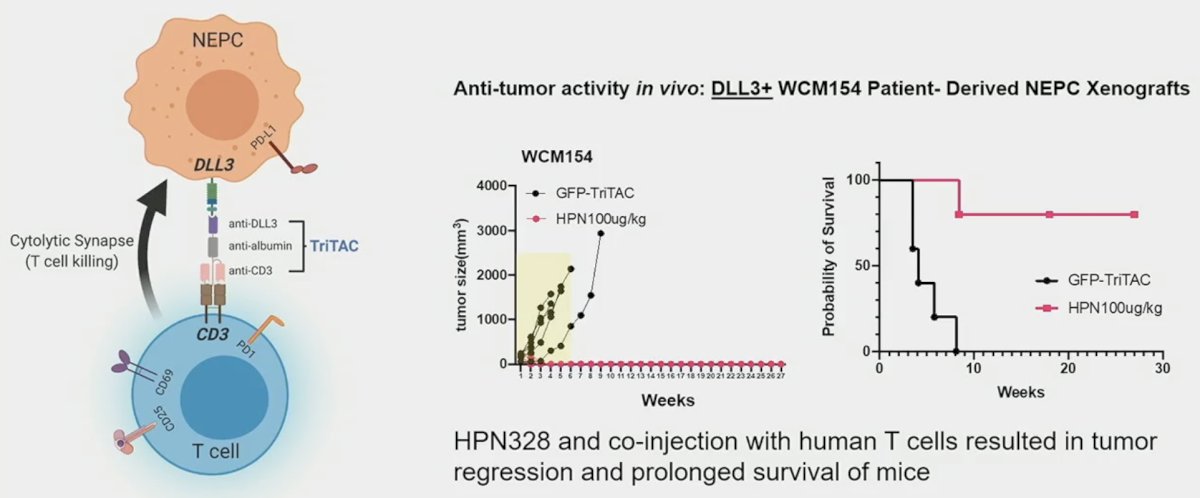
This phase 1/2 study employed a 3+3 dose escalation design for a total of 85 patients, of whom patients with neuroendocrine prostate cancer accounted for 15/85 (other tumors included small cell lung and other neuroendocrine cancers). HPN328 was administered weekly or bi-weekly.
The target population was:
- Neuroendocrine prostate cancer relapsed or refractory to standard of care
- Other DLL3+ high grade neuroendocrine neoplasms
- Small cell lung cancer relapsed/refractory to platinum chemotherapy
Dose optimization cohorts with 1 mg priming doses were planned:
- 6 mg once weekly
- 12 mg once weekly and every two weeks
- 24 mg once weekly and every two weeks
The trial objectives were to:
- Assess safety and tolerability
- Determine the recommended phase 2 dose (RP2D) and/or the maximum tolerated dose (MTD)
- Characterize pharmacokinetics and pharmacodynamics
- Evaluate preliminary anti-tumor activity
The baseline patient characteristics are summarized below. The median age of the GU neuroendocrine patients was 70 years. The median number of prior therapies was three, and the most common site of metastasis was the liver.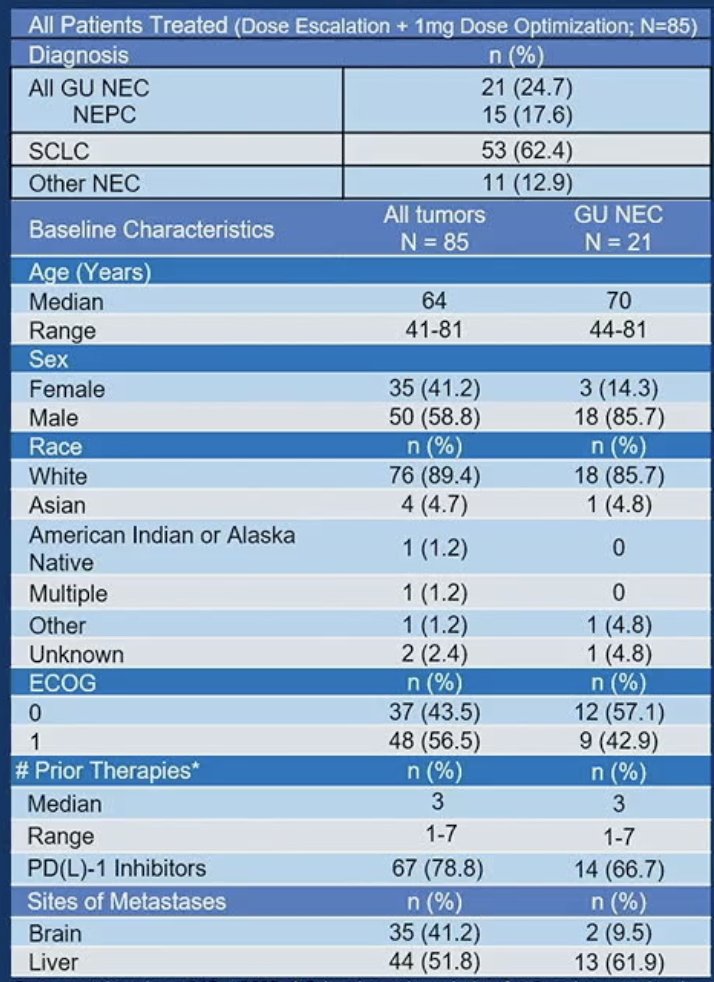
With regards to adverse/safety events in the overall cohort (n=85), two drug-limiting toxicities (DLTs) were seen at the priming dose of 2 mg (cytokine release syndrome); thus, the priming dose was de-escalated to 1 mg. No DLTs were observed at the target doses; target dose MTD was not reached. Grade ≥3 treatment-related adverse events were noted in 25% of patients. The most common were neutropenia (5%), cytokine release syndrome (3.5%), and diarrhea (2.4%).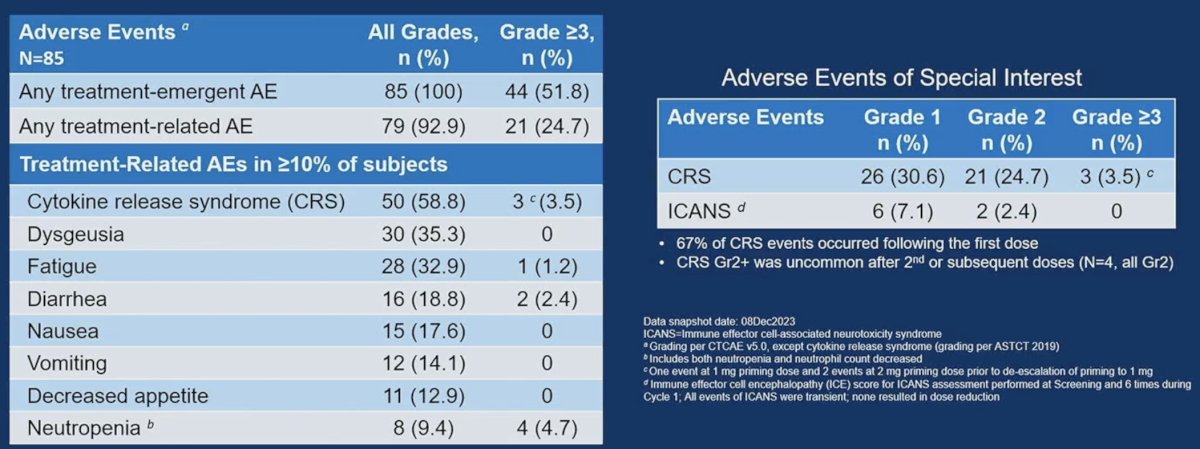
Anti-tumor activity in 1 mg priming subjects evaluable for response is summarized below. Among the 12 evaluable GU neuroendocrine cancer patients, the overall response rate was 58%, with a disease control rate of 83%.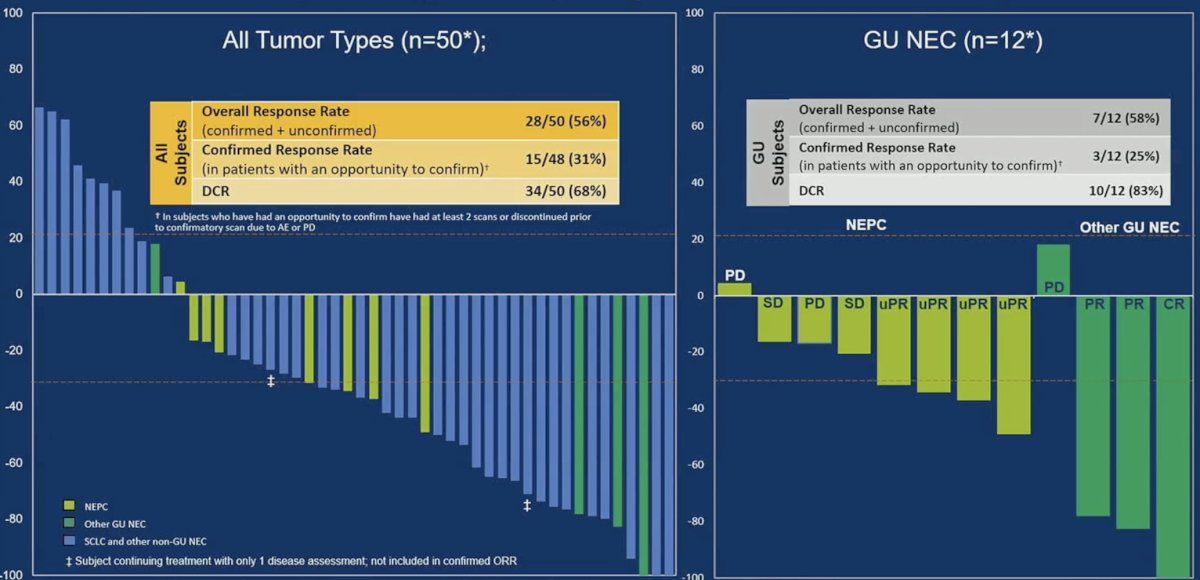
The swimmer’s plot below illustrates the duration of response and treatment among 17/21 GU neuroendocrine patients. One patient receiving 6 mg once weekly has an ongoing response at >50 weeks of treatment.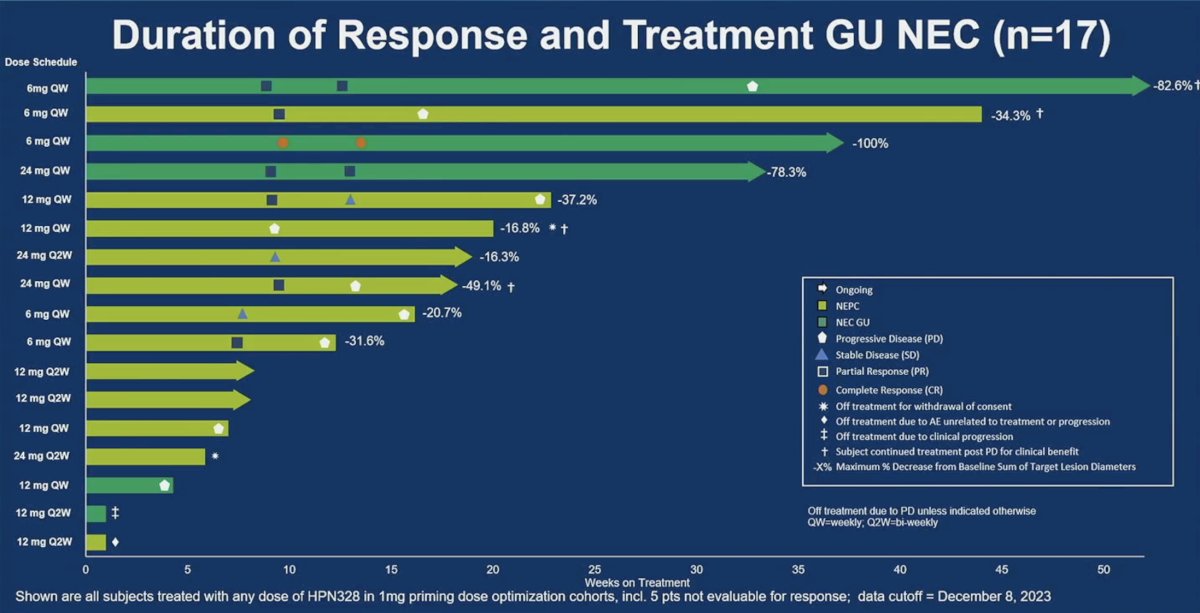
This patient presented with metastatic hormone-sensitive prostate cancer and a PSA of 35 ng/ml. He was treated with ADT + abiraterone/prednisone, and then 3.5 years later, developed new liver metastases, with a PSA of 0.08 ng/ml. A biopsy demonstrated high grade neuroendocrine prostate cancer. He subsequently progressed on carboplatin + cabazitaxel and was then switched to etoposide + durvalumab. He had evidence of progressive liver and bone metastases and was then enrolled on the HPN328 study. Notably, this patient had heterogenous DLL3 expression, as illustrated below:
Given this heterogeneity, was the clinical benefit driven by targeting more aggressive DLL3-positive clones? Nonetheless, this patient continues to have a response to treatment, highlighting HPN328’s activity in a heterogeneous expression setting.
With regards to DLL3 expression across tumor types and the need for testing, Dr. Beltran noted the following:
- For neuroendocrine prostate and small cell lung cancer, DLL3 pre-screening is not required
- Small cell bladder cancer (N=3); DLL3 expressed in 100% tumor cells
- For neuroendocrine prostate cancer:
- Potential sites of archival tissue, include the prostate, lymph node, liver, bone, pleura
- Neuroendocrine prostate cancer pathology: pure small cell carcinoma, high grade NEC, mixed adeno/small cell (with small cell component >80-90%)
- All 15 neuroendocrine prostate cancer patients had tumors positive for DLL3
- The range of DLL3 expression was 20 - 100% tumor cells
- There was no obvious correlation between %DLL3 positivity and response to therapy
- Were mixed responses due to DLL3 heterogeneity? Can a benefit be seen by targeting more aggressive DLL3 positive clones?
- Is there a potential for combination strategies?
- Additional biomarker analyses and clinical correlations are ongoing
Dr. Beltran concluded as follows:
- HPN328 is a novel DLL3 T cell engager that is well tolerated and demonstrates promising clinical activity in neuroendocrine carcinomas, including GU subtypes
- Adverse events were manageable, with cytokine release syndrome most commonly occurring with the first dose, mostly grades 1 or 2
- Monotherapy dose optimization is ongoing; maturing data in 12 and 24 mg cohorts will inform selection of the recommended phase 2 dose
Presented by: Himisha Beltran, MD, Associate Professor of Medicine in the Lank Center for Genitourinary Oncology and the Division of Molecular and Cellular Oncology, Director of Translational Research within Medical Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Reference:- Puca L, Gavyert K, Sailer V, et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med. 2019;11(484):eaav0891.


