(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer rapid oral abstract session. Dr. Amit Bahl presented the results of the ACE study, a multicentre prospective evaluation of cognitive function in patients with metastatic castrate-resistant prostate cancer (mCRPC) treated with abiraterone acetate or enzalutamide.
Both abiraterone acetate, in combination with prednisone/prednisolone, and enzalutamide are approved for the treatment of mCRPC patients in either the pre- or post-docetaxel settings.1-4 However, our understanding of the early impact of these treatments on the domains of cognitive impairment, fatigue, and depression in the real world remains limited. This prospective observational study was designed to bridge this knowledge gap and objectively assess patient-reported symptoms.
Cognition is the mental process of acquiring knowledge and understanding through thought, experience, and the senses. The six domains of cognitive function below were proposed in the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th edition, to help establish the etiology and severity of neurocognitive disorders.
- Attention and concentration: Ability to triage relevant information, thoughts, and actions while ignoring distractions; the ability to maintain attention for an extended period of time
- Executive function: Ability to initiate and generate hypotheses, to plan, and to make decisions.
- Information processing speed: Ability to quickly and efficiently process information.
- Visuospatial skill: Ability to process and interpret visual information about where things are in space.
- Language: Ability to comprehend and communicate symbolic information, both verbally and in writing.
- Learning and memory: Ability to learn new information; ability to store and recall new information, in either the short term or the long term
In prostate cancer, a triad has been theorized to explain the observed increased prevalence of cognitive dysfunction:
- Patient factors, including age, innate genetic risk, functional status, and biologic markers
- Polypharmacy: Cancer therapies and co-medications for non-cancer comorbidities
- Disease burden: Prostate cancer and comorbid conditions

This was a non-randomized, observational prospective study that included mCRPC patients selected for treatment with either enzalutamide or abiraterone acetate. Patients were subsequently registered into the ACE trial and underwent CANTAB cognitive assessment. Choice of abiraterone or enzalutamide was at the treating physician’s discretion Patients being treated with either drug as part of another clinical trial or study, and those clinically diagnosed with dementia were deemed study ineligible.
The cognitive application used was CANTAB, an intuitive touchscreen interface tool that digitally assesses all cognitive domains. The assessed time points were:
- Baseline
- 3 to 4 months on treatment
- 6 months on treatment
- 12 months on treatment

The study cohort included 253 patients from 12 participating sites, of whom 141 and 112 received abiraterone acetate and enzalutamide, respectively. The median patient age was 74 years. Almost 50% of patients had received prior docetaxel. There were 184 and 131 respondents at the 3-4 months and 6 months follow-up time points. In this analysis, Dr. Bahl reported the data for these two time points.
After controlling for baseline levels, no differences were observed between the abiraterone acetate- and enzalutamide-treated patients with regards to mean composite cognitive
outcome (3 – 4 months: p=0.55, 6 months: p=0.2) or for the individual components for Spatial Working Memory, Rapid Information Processing, or Spatial Information Processing.
The difference in Reaction Time Task was significant at 3-4 months (p=0.009) and 6 months (p=0.037), with significant deterioration in the enzalutamide group.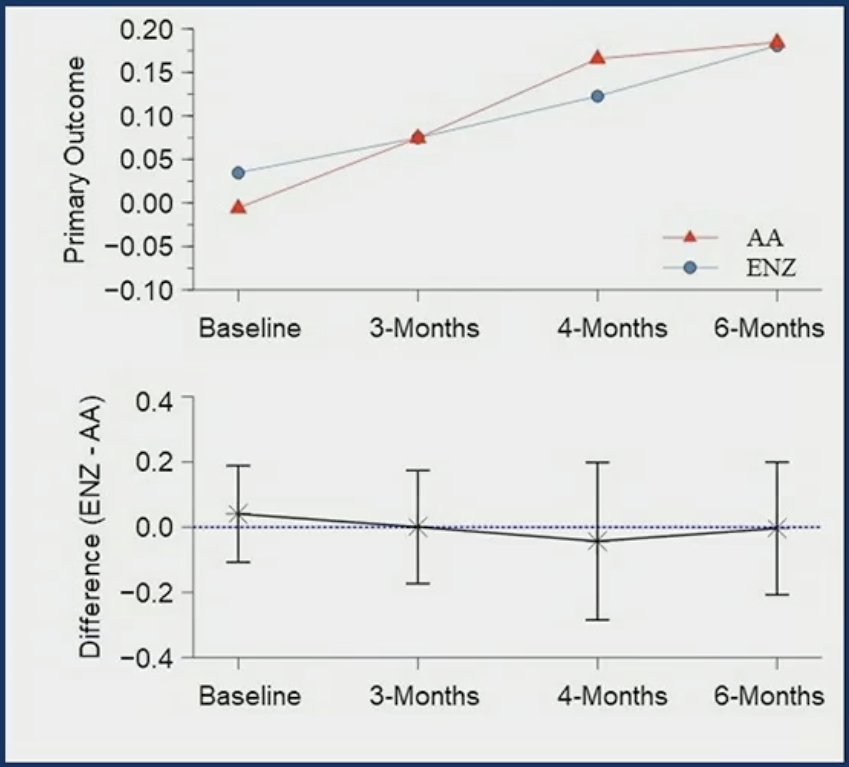
With regards to patient-reported outcomes, mean fatigue changes (FACT-F scale) were observed between baseline and each subsequent timepoint for enzalutamide-treated patients (p< 0.001), but not for those in the abiraterone group. There was note of a statistically significant difference in mean fatigue between abiraterone and enzalutamide-treated patients at 3-4 months (p<0.001) and at 6 months (p <0.001).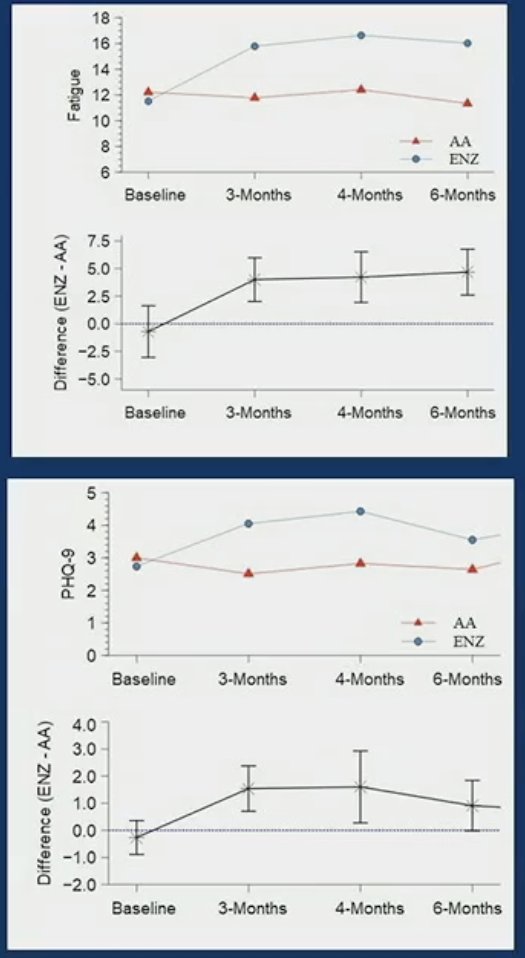
Dr. Bahl concluded that this study shows that while composite cognitive outcome is comparable in mCRPC patients treated with abiraterone or enzalutamide at 3 – 4 and 6 months, patients on enzalutamide report worse fatigue, depression, and deterioration in perceived cognitive ability and have a slower reaction time when compared to abiraterone acetate. This is an important consideration for treatment optimization and ensuring supportive strategies for mCRPC patients when using these drugs.
Presented by: Amit Bahl, MD, FRCP, FRCR, Bristol Haematology and Oncology Centre, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424-433.


