(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Amit Raval discussing real-world utilization patterns of radium-223 in metastatic prostate cancer in the United States. Radium-223 is approved for use in men with symptomatic castration-resistant prostate cancer (CRPC) with bone metastases, demonstrating benefits in terms of overall survival and quality of life with a reliable safety profile.1 The phase 3 ALSYMPCA trial,1 was a phase 3, randomized, double-blind, placebo-controlled trial that randomized 921 patients in a 2:1 fashion to receive six injections of radium-223 or matching placebo. The primary endpoint was overall survival and patients receiving radium-223 had significantly improved median overall survival (14.9 versus 11.3 months; HR 0.70, 95% CI 0.58 to 0.83). In the rapidly evolving treatment landscape for prostate cancer, real world data on radium-223 utilization are limited. Dr. Raval and colleagues aimed to examine the real-world utilization patterns of radium-223 in patients with metastatic prostate cancer using a large administrative claims database in the United States.
This was a retrospective cohort of men treated with radium-223 identified using the closed claims private payor database of the Komodo Research Dataset (KRD) covering the period from January 1, 2017 to June 30, 2022:
KRD is a verified, adjudicated, and de-identified administrative claims dataset of over 140 million individuals from over 150 private insurance providers, including commercial, Medicaid Managed Care, and Medicare Advantage plans. The KRD represents the US population covered through private insurance.
The earliest administration of radium-223 in men with bone metastatic prostate cancer was considered as index date. Individuals were required to have continuous insurance coverage at least 12-month pre-index (baseline) and least 6-month post index or until death if patients survived < 6 months (follow-up). The study outcome was completion of 5+ cycles of radium-223, identified as 5+ distinct medical claims with procedure codes for radium-223 administration without a gap of >=56 days between any two sequential treatments. Logistic regression was used to identify baseline demographic, clinical, medications, or healthcare resource use-related factors associated with receiving 5+ cycles of radium-223.
The study cohort comprised 1,376 men with a median age of 68 years at the time of radium-223 initiation. Most men were treated by oncologists (52.2%), having bone only metastases (51.5%), and having Charlson comorbidity index (CCI) ≥ 1 (76.2%). Diabetes (34.1%), peripheral vascular disease (29.9%), pulmonary disease (25.9%) and mild liver disease (25.1%) were the most common pre-existing comorbidities:

Therapies received prior to radium-223 were:
- Androgen receptor pathway inhibitors (75.4%)
- Chemotherapy (32.1%)
- Sipuleucel-T (13.7%)
- Bone health agents (73.7%)
Radium-223 was utilized as a combination therapy by 26.0% of the overall population, predominately with enzalutamide. Nearly 15% received radium-223 (monotherapy or combination therapy) as first line therapy, 38% as second line therapy, and 31% as third line therapy. The proportion of men who completed 5+ cycles of radium-223 were 46% and 54% in the overall cohort and the subset of men who survived >=6 months:

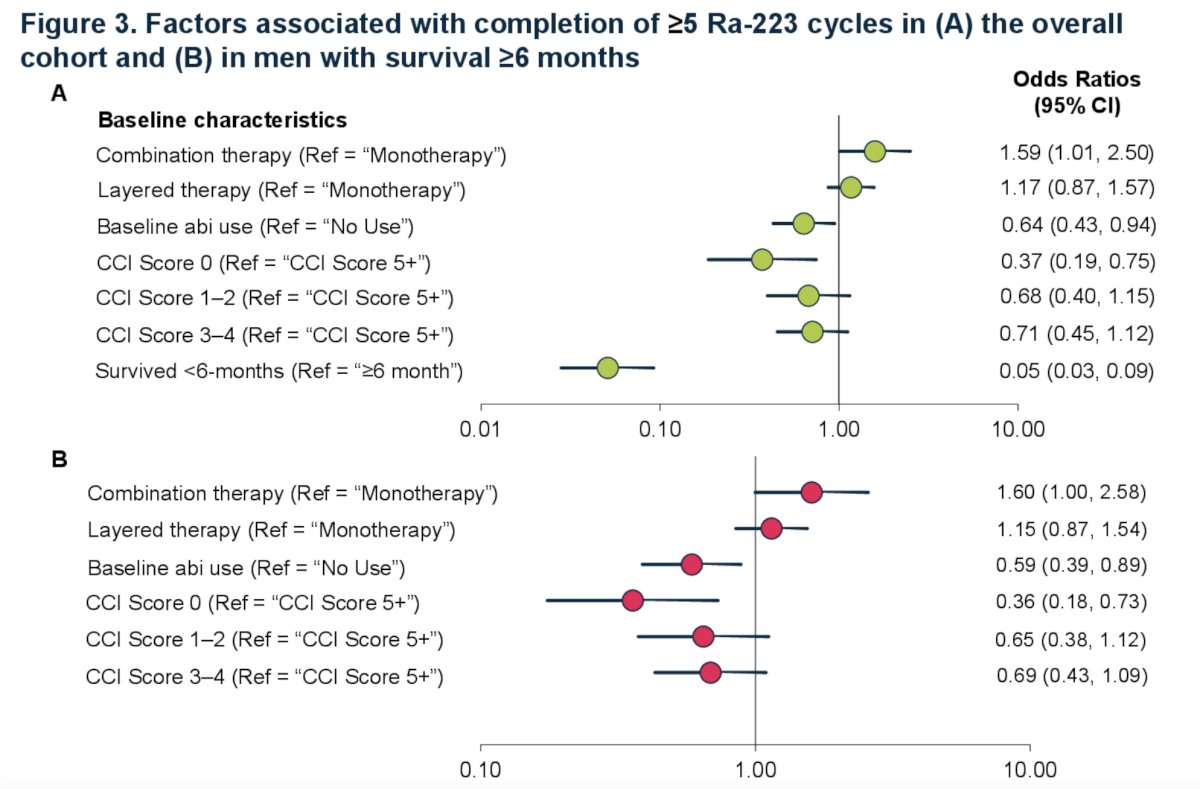
In regression analysis of the overall cohort, men with index combination therapy were more likely than monotherapy to receive 5+ cycles (OR 1.59, 95% CI 1.01 – 2.50). Men with short survival (<6 month) (OR 0.05, 95% CI 0.03 - 0.09), low CCI (0 vs. 4+) (OR 0.37, 95% CI 0.19 - 0.75), and prior abiraterone use (OR 0.64, 95% CI 0.43 - 0.94) had lower likelihood of completion of 5+ cycles compared to their counterparts. These findings were also consistent with a subset of men with survival >= 6 months:
Dr. Raval concluded his presentation discussing real-world utilization patterns of radium-223 in metastatic prostate cancer in the United States with the following take-home points:
- This large study is the first of its kind to assess utilization patterns of radium-223 representative of private insurance enrollees within the United States
- Nearly half of men completed 5+ cycles of radium-223 therapy in a large US cohort of relatively young mCRPC, with completion more likely in men who received radium-223 as combination therapy
- Advanced disease state with short survival and low comorbidity were key factors associated with lower completion of 5+ cycles
- The findings highlight the importance of utilizing radium-223 early after mCRPC diagnosis, when the probability of >6-month survival is higher, to enhance treatment completion that has been shown to result in better outcomes
Presented by: Amit D. Raval, PhD, Bayer HealthCare Pharmaceuticals, Whippany, NJ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
1. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.


