(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer rapid oral abstract session. Dr. Scott Eggener presented SEPTA, a multicenter, prospective validation trial of Stockholm 3 for prostate cancer in a multi-ethnic cohort.
Dr. Eggener noted that current PSA-based prostate cancer screening approaches may reduce prostate cancer mortality,1 but may be associated with numerous harms, most notably cancer overdiagnosis and subsequent overtreatment. Accordingly, there has been increased interest in evaluating alternative screening approaches to minimize such harms, while maintaining the potential benefits of screening and early diagnosis.
Stockholm3 is a blood-based diagnostic test that combines the following parameters:
- Proteins
- Human glandular kallikrein 2 (hK2)
- Microseminoprotein beta (MSMB)
- Microphage inhibitory cytokine-1 (MIC1)
- Total PSA
- Free PSA
- Genetic markers
- 101 Single nucleotide polymorphisms
- Clinical data
- Age
- Family history
- Previous prostate biopsy

Classically a Stockholm3 risk score of at least 11% (for diagnosing Grade Group ≥2 disease) is considered an indicator of increased prostate cancer risk, however, higher cut-offs (e.g., 15% or 17%) are being increasingly used. This biomarker has been evaluated in population-based randomized controlled trials in Sweden and has been shown to reduce the harms associated with PSA-based screening, most notably by reducing the frequency of ‘over-biopsying’.2 However, the Swedish population is a highly homogenous one and may not be representative of other geographic populations. As such, external validation of this biomarker in racially/ethnically diverse populations is needed.
This study prospectively recruited patients from 2019 to 2023 at 17 sites (n=912) in both the United States and Canada, and additionally included men who were previously biopsied and had bio-banked blood (n=1,217) for a total of 2,129 men. The eligibility criteria were as follows:
- No known prostate cancer
- Having clinical indications for a prostate biopsy
- Self-identified as:
- Asian
- Black or African American
- Hispanic or Latino
- Non-Hispanic non-Latino and White
- Ages 45 – 75 years
- No urologic instrumentation or digital rectal examination immediately prior to the blood draw
- PSA, Stockholm3, and biopsy outcome data available
Overall, of the 2,129 included men, 1,160 (55%) identified as being racial/ethnic minorities. The primary study endpoint was diagnosis of Grade Group ≥2 prostate cancer, with a secondary endpoint of diagnosing benign or Grade Group 1 disease. The primary study objectives were as follows:
- To evaluate the relative sensitivity for detection of Grade Group ≥ 2 prostate cancer using Stockholm3 (≥15) compared to PSA (≥4 ng/ml), with a non-inferiority margin of 0.8
- To evaluate the relative specificity for the avoidance of benign or Grade Group 1 cancer using Stockholm3 (≥15) compared to PSA (≥4 ng/ml)
- Sub-group analyses were performed for the primary aims in each of the race and ethnicity subgroups
The median patient age was 63 years, with a median PSA of 6.1 ng/ml (IQR: 4.5 – 9.0). 20% of patients had a prior negative biopsy. The median Stockholm3 score was 17% (IQR: 10 – 30).
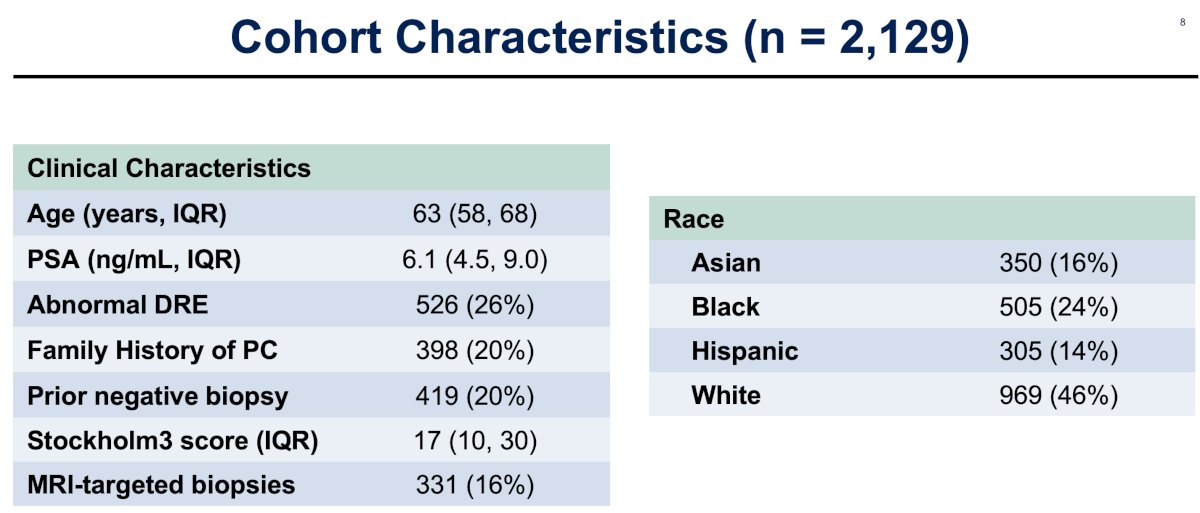
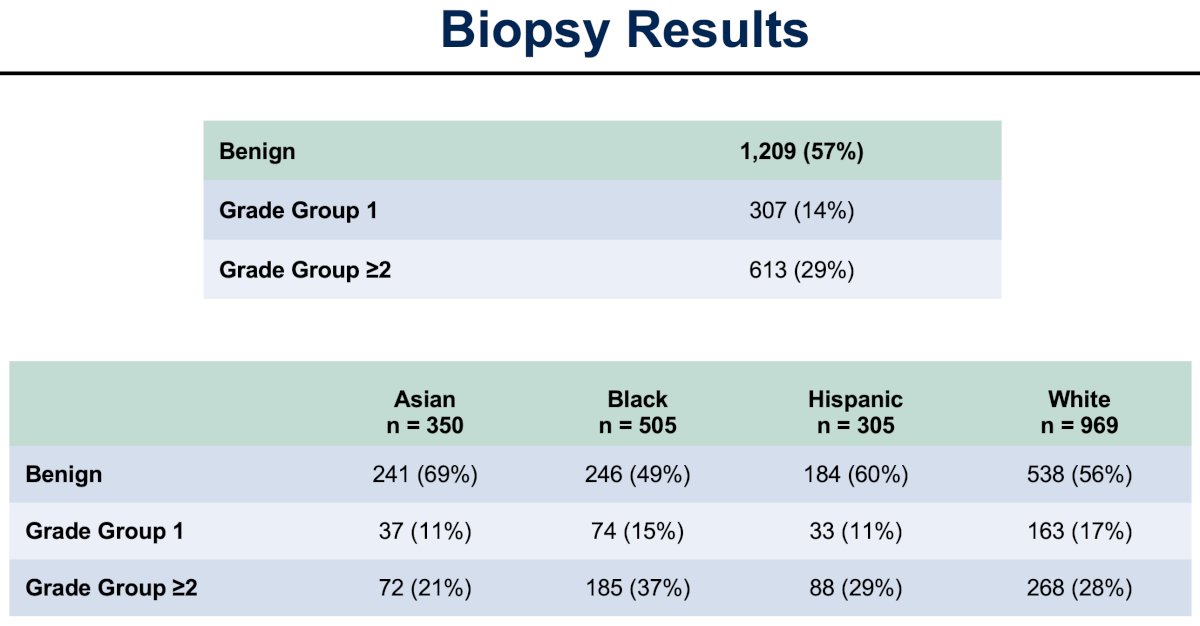
Notably, 16% of patients had MRI-targeted biopsies. Of the 331 biopsies performed, 14% and 29% demonstrated Grade Group 1 and Grade Group ≥2 disease, respectively. The relative frequency of Grade Group ≥2 disease by race/ethnicity was highest in Black men (37%), versus Hispanic (29%), White (28%), and Asian men (21%).
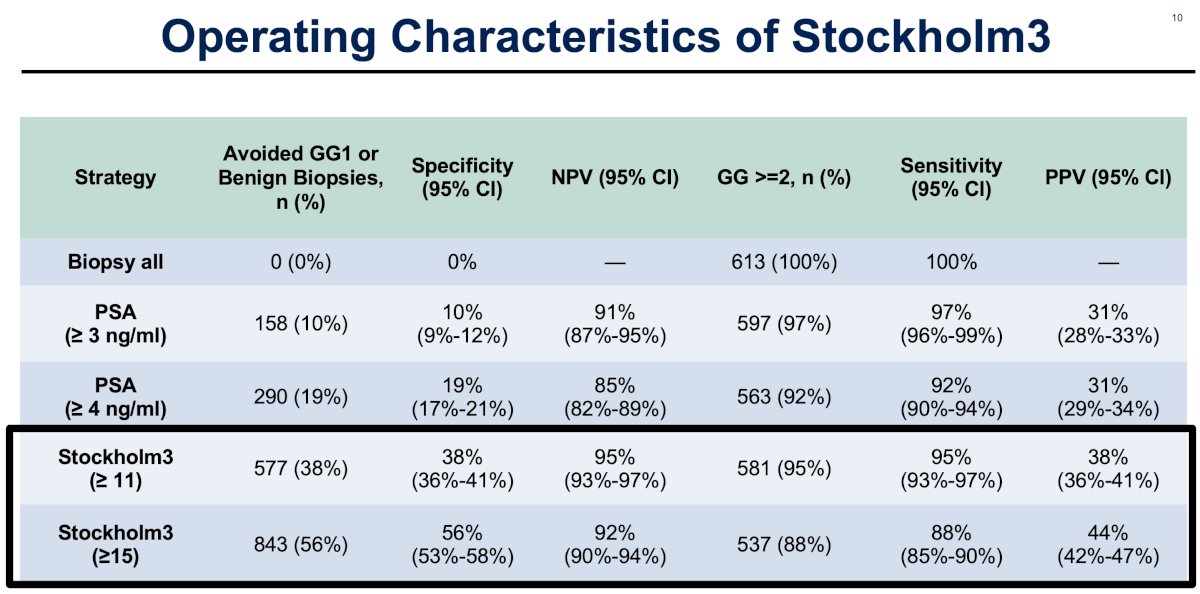
The Stockholm3 test, irrespective of whether an 11% or 15% cut-off was used, outperformed a PSA cut-off of ≥4 ng/ml for avoiding a benign biopsy or Grade Group 1 diagnosis (38 – 56% versus 19%). Accordingly, the specificity (38 – 56% versus 19%) and negative predictive values (92 – 95% versus 85%) were also superior for Stockholm3. The sensitivity of Stockholm3 at the cut-offs of 11% and 15% (95% and 88%, respectively) was similar to that of a PSA ≥4 ng/ml (92%).
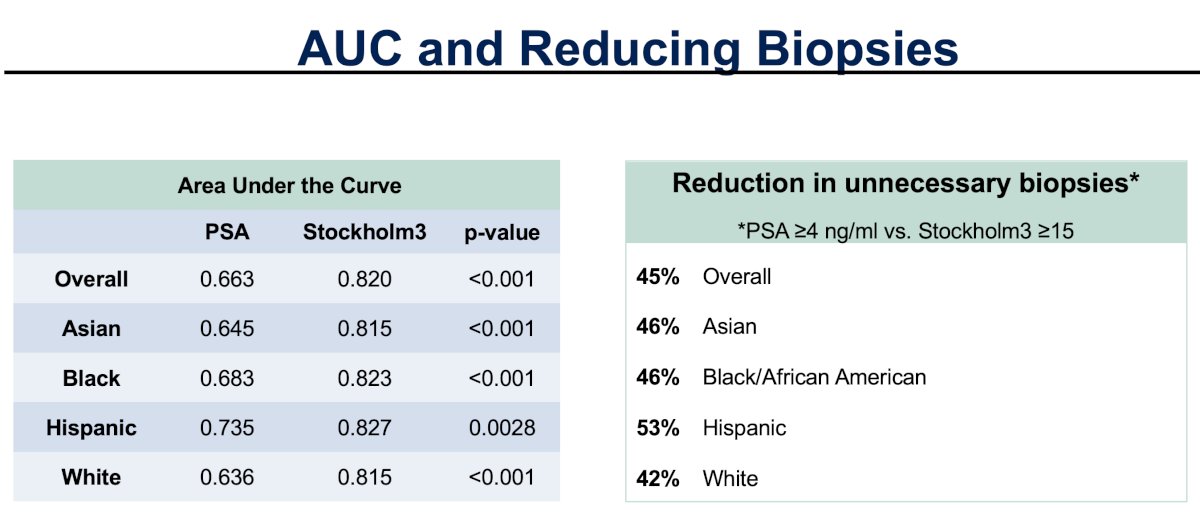
When evaluated in the overall cohort and by race/ethnicity, Dr. Eggener and colleagues observed that the relative sensitivity of a Stockholm3 ≥ 15 cut-off was non-inferior to a PSA ≥4 ng/ml (the relative sensitivity did not cross the lower boundary of 0.80 for any of the racial/ethnic groups). Furthermore, the relative specificity of Stockholm3 ≥ 15 exceeded that of PSA ≥4 ng/ml across all racial/ethnic subgroups.

Overall, a Stockholm3 ≥15 cut-off reduced unnecessary biopsies by 45%, compared to a PSA ≥4 ng/ml. The corresponding overall area under the curve (AUC) values were 0.82 and 0.663, respectively.
Dr. Eggener concluded that:
- The use of Stockholm3 has the potential to reduce unnecessary harms of prostate cancer screening
- Stockholm3 has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups
- This study represents a successful recruitment of a large cohort of underrepresented minorities to a prostate cancer trial
Presented by: Scott E. Eggener, MD, Bruce and Beth White Family Professor of Surgery, Department of Urology, University of Chicago, Chicago, IL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Hugosson J, Roobol MJ, Mansson M, et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur Urol. 2019;76(1):43-51.
- Strom P, Nordstrom T, Aly M, et al. The Stockholm-3 Model for Prostate Cancer Detection: Algorithm Update, Biomarker Contribution, and Reflex Test Potential. Eur Urol. 2018;74(2):204-10.


