(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Sreevalsa Appukkuttan discussing clinical and patient factors associated with treatment intensification for metastatic hormone-sensitive prostate cancer (mHSPC). Multiple trials have shown that intensified therapies convey a survival advantage over ADT alone in mHSPC. This study presented at GU ASCO 2024 sought to identify area- and patient-level factors that are associated with the intensification of therapy among mHSPC patients treated in US health systems.
Electronic health records from OPTUM were used to identify patients aged ≥18 with mHSPC who received ADT between 2020 and 2022. Treatment intensification was defined as receiving one of the following within 90 days of initiation of ADT:
- Apalutamide
- Enzalutamide
- Abiraterone +/- prednisone +/- docetaxel
- Darolutamide +/- docetaxel
- Docetaxel +/- other systemic therapy
- External beam radiation therapy
Multivariable logistic regression was used to determine factors associated with intensification.
This study identified 1,123 patients with mHSPC who received ≥1 ADT in the post-index period with mean age of 71.2 (SD 9.5). The cohort comprised 73% Caucasians, 18% African Americans, and 2% Asians. Overall, 640 (57%) patients received treatment intensification within 90 days of ADT initiation. Importantly, the proportion of patients receiving intensification increased from 53% to 63% between 2020 and 2022. The most common intensification treatments were abiraterone acetate (46%) and enzalutamide (38%). The median time to first treatment after metastasis was 15 days for receiving intensified therapy and 34 days for non-intensified therapy. In multivariable logistic regression, younger age, presence of bone/bone marrow metastasis at baseline, region (Northeast and West), and lower modified Charlson Comorbidity Index score (excluding cancer) were significantly associated with receiving intensified therapy:
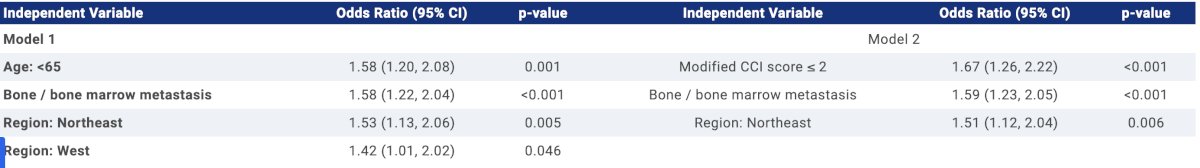
PSA was not included in the models due to 40% of patients missing data.
Dr. Appukkuttan concluded this presentation discussing clinical and patient factors associated with treatment intensification for mHSPC with the following take-home points:
- Just over half of eligible patients received treatment intensification following ADT
- There are also significant disparities in treatment by age, region, and site of metastases
- Future studies examining physician and patient preferences for intensification, as well as possible misperceptions and biases, would provide evidence to address under treatment in this setting
Presented by: Sreevalsa Appukkuttan, MPH, MBBS, Bayer HealthCare Pharmaceuticals Inc, Whippany, NJ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


