(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Sagar Patel presented the initial analysis of patient treatment preferences and quality of life from the REVELUTION trial, comparing relugolix to leuprolide in combination with radiotherapy for the treatment of localized prostate cancer patients.
Relugolix is a novel oral gonadotropin releasing hormone (GnRH) antagonist that was demonstrated in the non-inferiority phase 3 HERO trial to have superior testosterone suppression (faster suppression and deeper responses) and possibly lower cardiovascular toxicity compared to the injectable LHRH agonist, leuprolide, which is more commonly used in the United States.1
The Relugolix Versus Leuprolide Trial (REVELUTION; NCT 05320406) is an ongoing, investigator-initiated, open-label randomized controlled trial comparing radiotherapy + relugolix versus radiotherapy + leuprolide for men with localized prostate cancer. Notably, men treated with radiotherapy alone without ADT were allowed to opt for enrolment on a parallel cohort control arm.
The primary study endpoint was a change in coronary plaque on cardiac computed tomography (CT) from baseline to 12 months following treatment. Secondary endpoints included patient-reported toxicity, with an exploratory endpoint of patient treatment preference between leuprolide and relugolix.
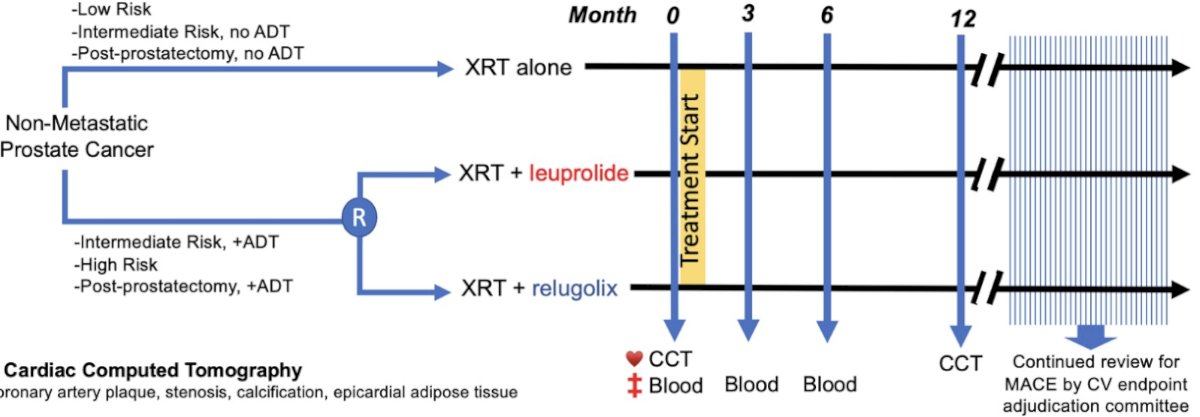
Eligible patients were men with non-metastatic intact or biochemically recurrent prostate cancer who were scheduled for pelvic radiotherapy (1.8 – 2.5 Gy per fraction) with planned ≥6 months of ADT. These patients underwent 1:1 randomization to leuprolide (intramuscularly/subcutaneously at 22.5 mg every 3 months or 45 mg every 6 months) versus relugolix (orally at 360 mg day 1, 120 mg daily thereafter). Prior to randomization, each patient completed an ADT preference questionnaire after reviewing an education brochure describing both drugs' administration, testosterone kinetics, and common toxicities.
Patient-reported toxicity was assessed by the International Prostate Symptom Score
(IPSS), Sexual Health Inventory Metric (SHIM), and Expanded Prostate Index Composition for Clinical Practice (EPIC-CP) at baseline and 3- to 6-months intervals following radiotherapy initiation. Given the repeated measures nature within the same patients followed longitudinally, the scores were compared using linear mixed-effects models accounting for within-subject correlations.
Between June 2022 and July 2023, 65 men planned for treatment with radiotherapy + ADT were randomized to either leuprolide (n=34) or relugolix (n=31). The parallel cohort control arm included 26 men treated with radiotherapy alone. The median study follow-up was 5 months (IQR: 4 – 8), and the median patient age was 67 years (IQR: 64 – 73 years).
Compared to patients receiving leuprolide + radiotherapy, those receiving relugolix + radiotherapy were significantly less impacted on IPSS urinary score (mean difference [MD]: -3.68, p=0.04). There were no between group differences in EPIC-CP overall (MD: -1.82), incontinence (MD: +0.07), bowel (MD: +0.39), sexual (MD: -0.74), or vitality (MD: +0.17). There were similarly no differences in SHIM (MD: +1.27) scores.

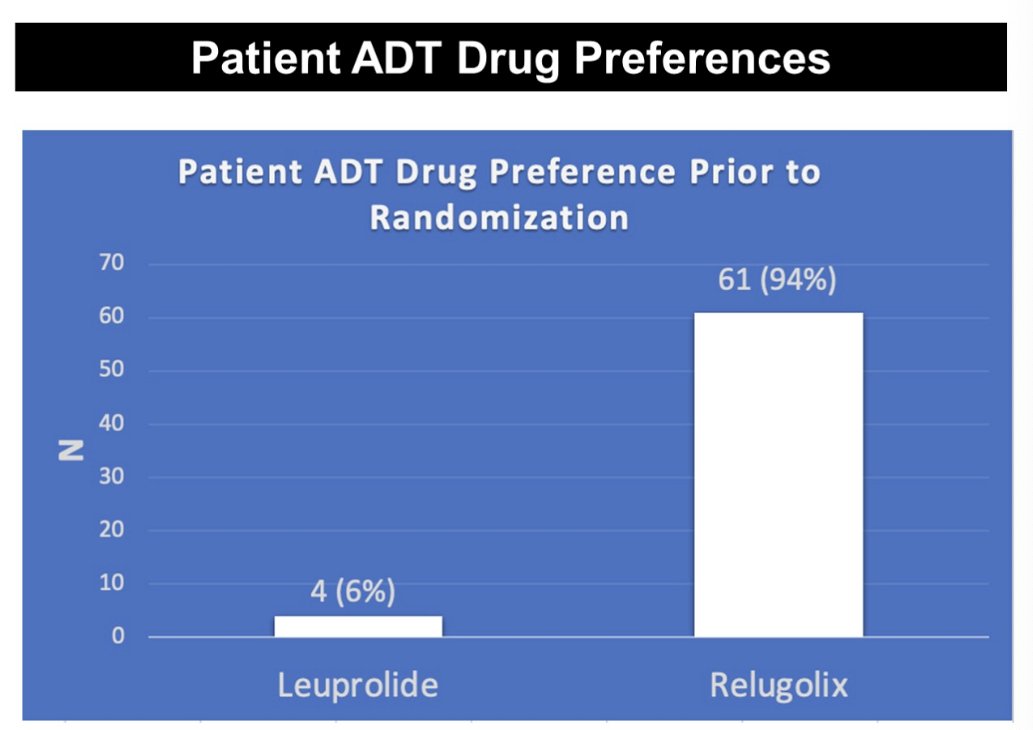
The majority of patients (94%) preferred receipt of relugolix, as opposed to leuprolide, prior to randomization. The most commonly cited reasons were the testosterone kinetics (59%), potential lower cardiovascular risk (38%), and the oral route of administration (3%).
Dr. Patel concluded as follows:
- REVELUTION is an ongoing single institution, open-label, randomized trial comparing leuprolide versus relugolix in combination with radiotherapy in men with localized prostate cancer
- If given the choice outside of a randomization, most patients preferred relugolix over leuprolide (assuming cost-neutral)
- Relugolix, compared with leuprolide, mitigated acute urinary toxicity from radiotherapy, perhaps due to superior castration resulting in greater prostate gland downsizing and less obstructive uropathy
- Longer-term follow-up is ongoing; primary cardiovascular toxicity endpoints are expected to be complete in late 2024
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


